Meta-Analysis of Randomized Controlled Trials on Gamma-Aminobutyric Acid Analogues and Opioid-Based Therapies for CKD-Associated Pruritus
IF 5.7
2区 医学
Q1 UROLOGY & NEPHROLOGY
引用次数: 0
Abstract
Introduction
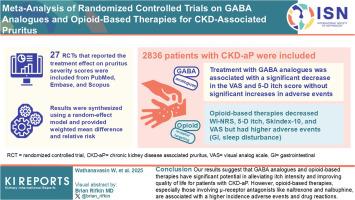
Chronic kidney disease (CKD)-associated pruritus (CKD-aP) is one of the most distressing symptoms of patients with CKD, adversely affecting their quality of life and survival. This study aimed to investigate the efficacy and safety of gamma-aminobutyric acid (GABA) analogues and opioid-based therapies in patients with CKD-aP.
Methods
The eligible studies were searched from PubMed, Embase, and Scopus up to January 22, 2025. Only randomized controlled trials (RCTs) that reported the treatment effect on pruritus severity scores were included, focusing on both unidimensional scales and multidimensional scales. The results were synthesized using a random-effect model and provided weighted mean difference (WMD) and relative risk (RR) with a 95% confidence interval (CI).
Results
A total of 27 RCTs involving 2836 patients with CKD-aP were analyzed. Treatment with GABA analogues was associated with significantly greater reductions in 10-cm visual analog scale (VAS) and 5-D itch scores with nonsignificant increased incidence of adverse events (AEs) or adverse drug reactions (ADRs). In addition, opioid receptor–targeting treatments significantly decreased worst itch numeric rating scale (WI-NRS), 5-D itch, Skindex-10, and 100-mm VAS, but showed significantly higher rates of AEs or ADRs, particularly related to gastrointestinal issues, as well as neurological disorders such as sleep disturbances.
Conclusion
Our results suggest that GABA analogues and opioid-based therapies, particularly difelikefalin, have significant potential in alleviating itch intensity and improving the quality of life of patients with CKD-aP. However, opioid-based therapies, especially those involving μ-receptor antagonists such as naltrexone and nalbuphine, are associated with a higher incidence of AEs or ADRs. Given the substantial heterogeneity observed in most of the results, interpretation should be approached with caution.
-氨基丁酸类似物和阿片类药物治疗ckd相关瘙痒的随机对照试验荟萃分析
慢性肾脏疾病(CKD)相关性瘙痒(CKD- ap)是CKD患者最痛苦的症状之一,对他们的生活质量和生存产生不利影响。本研究旨在探讨γ -氨基丁酸(GABA)类似物和阿片类药物治疗CKD-aP患者的疗效和安全性。方法检索截至2025年1月22日的PubMed、Embase和Scopus中符合条件的研究。仅纳入报告瘙痒严重程度评分的治疗效果的随机对照试验(rct),重点是一维量表和多维量表。结果采用随机效应模型进行综合,并提供加权平均差(WMD)和相对风险(RR),置信区间为95%。结果共分析了27项随机对照试验,涉及2836例CKD-aP患者。GABA类似物治疗与10厘米视觉模拟量表(VAS)和5-D瘙痒评分的显著降低相关,而不良事件(ae)或药物不良反应(adr)的发生率无显著增加。此外,阿片受体靶向治疗显著降低了最严重瘙痒数值评定量表(WI-NRS)、5-D瘙痒、skinindex -10和100毫米VAS,但显示出明显更高的ae或adr发生率,特别是与胃肠道问题以及神经系统疾病(如睡眠障碍)有关。我们的研究结果表明,GABA类似物和基于阿片类药物的治疗,特别是difelikefalin,在缓解CKD-aP患者的瘙痒强度和改善生活质量方面具有显著的潜力。然而,以阿片类药物为基础的治疗,特别是那些涉及μ受体拮抗剂如纳曲酮和纳布啡的治疗,与较高的不良反应或不良反应发生率相关。鉴于在大多数结果中观察到的实质性异质性,解释应谨慎进行。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Kidney International Reports
Medicine-Nephrology
CiteScore
7.70
自引率
3.30%
发文量
1578
审稿时长
8 weeks
期刊介绍:
Kidney International Reports, an official journal of the International Society of Nephrology, is a peer-reviewed, open access journal devoted to the publication of leading research and developments related to kidney disease. With the primary aim of contributing to improved care of patients with kidney disease, the journal will publish original clinical and select translational articles and educational content related to the pathogenesis, evaluation and management of acute and chronic kidney disease, end stage renal disease (including transplantation), acid-base, fluid and electrolyte disturbances and hypertension. Of particular interest are submissions related to clinical trials, epidemiology, systematic reviews (including meta-analyses) and outcomes research. The journal will also provide a platform for wider dissemination of national and regional guidelines as well as consensus meeting reports.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


