The assessment of interaction kinetics between specific monoclonal antibody and immobilized SARS-CoV-2 nucleoprotein
IF 4.5
2区 化学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
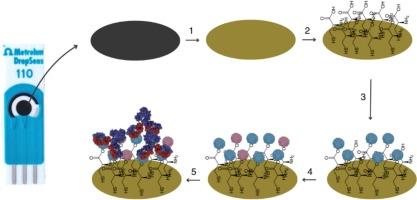
The emergence of SARS-CoV-2 has posed significant global health challenges. The nucleocapsid protein (N-protein) is a structural part of the SARS-CoV-2 virus and an important immunogenic target of specific antibodies, which are developed in the organism during the infection by this virus. Artificially designed specific (monoclonal and polyclonal) antibodies are also used for therapeutic and bioanalytical purposes, therefore, the assessment and characterization of newly designed antibodies is an important analytical issue. This study reports an electrochemical biosensing system for the assessment of the interaction between newly designed specific antibody and SARS-CoV-2 recombinant N-protein, against which the antibody was designed, and which was used as a target in biological recognition part of the biosensing system. The biosensing system was applied for the determination of the interaction kinetics between immobilized N-protein and a newly derived monoclonal antibody (mAb) 16D9 (mAb-16D9). Cyclic voltammetry was employed to evaluate the kinetics of the interaction between the recombinant N-protein and mAb-16D9. The binding constant (Kc) was determined to be 50.99 μg/mL, demonstrating a strong affinity, while the limit of detection was 4.3 × 10−4 μg/mL, highlighting sufficient affinity of mAb-16D9 towards N-protein, which determined good sensitivity of the developed biosensors. These findings highlight the potential application of the here-reported electrochemical biosensor for rapid testing of antigen-antibody interaction kinetics and the characterization of newly designed antibody.
特异性单克隆抗体与固定化SARS-CoV-2核蛋白相互作用动力学评价
SARS-CoV-2的出现构成了重大的全球卫生挑战。核衣壳蛋白(n蛋白)是SARS-CoV-2病毒的结构组成部分,是机体在感染该病毒过程中产生的特异性抗体的重要免疫原性靶点。人工设计的特异性(单克隆和多克隆)抗体也用于治疗和生物分析目的,因此,新设计抗体的评估和表征是一个重要的分析问题。本研究报道了一种电化学生物传感系统,用于评估新设计的特异性抗体与SARS-CoV-2重组n蛋白之间的相互作用,并将其作为生物传感系统生物识别部分的靶点。应用该生物传感系统测定了固定化n蛋白与新获得的单克隆抗体(mAb) 16D9 (mAb-16D9)的相互作用动力学。采用循环伏安法评价重组n蛋白与mAb-16D9相互作用的动力学。结合常数(Kc)为50.99 μg/mL,具有较强的亲和力,检出限为4.3 × 10−4 μg/mL,表明单克隆抗体- 16d9对n蛋白具有较强的亲和力,具有较好的灵敏度。这些发现突出了本文报道的电化学生物传感器在快速检测抗原-抗体相互作用动力学和新设计抗体表征方面的潜在应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioelectrochemistry
生物-电化学
CiteScore
9.10
自引率
6.00%
发文量
238
审稿时长
38 days
期刊介绍:
An International Journal Devoted to Electrochemical Aspects of Biology and Biological Aspects of Electrochemistry
Bioelectrochemistry is an international journal devoted to electrochemical principles in biology and biological aspects of electrochemistry. It publishes experimental and theoretical papers dealing with the electrochemical aspects of:
• Electrified interfaces (electric double layers, adsorption, electron transfer, protein electrochemistry, basic principles of biosensors, biosensor interfaces and bio-nanosensor design and construction.
• Electric and magnetic field effects (field-dependent processes, field interactions with molecules, intramolecular field effects, sensory systems for electric and magnetic fields, molecular and cellular mechanisms)
• Bioenergetics and signal transduction (energy conversion, photosynthetic and visual membranes)
• Biomembranes and model membranes (thermodynamics and mechanics, membrane transport, electroporation, fusion and insertion)
• Electrochemical applications in medicine and biotechnology (drug delivery and gene transfer to cells and tissues, iontophoresis, skin electroporation, injury and repair).
• Organization and use of arrays in-vitro and in-vivo, including as part of feedback control.
• Electrochemical interrogation of biofilms as generated by microorganisms and tissue reaction associated with medical implants.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


