Thymoquinone ameliorates cognitive impairment and neuroinflammation in an amyloid-beta-induced rat model of Alzheimer’s disease
IF 2.6
4区 医学
Q3 NEUROSCIENCES
引用次数: 0
Abstract
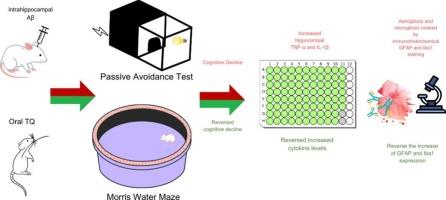
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder characterized by cognitive decline and memory impairment. Amyloid-beta (Aβ) peptide accumulation is one of the most important chatacteristics of AD that cause neuronal damage and neuroinflammation. Thymoquinone (TQ), a bioactive compound derived from Nigella sativa, has shown neuroprotective properties in previous studies. This study aimed to evaluate the ameliorative effects of TQ in an Aβ1-42-induced AD rat model. Male Wistar-Albino rats were divided into four groups: Control, AD, TQ-10 (10 mg/kg TQ), and TQ-30 (30 mg/kg TQ). TQ was administered orally for 7 days before and 10 days after Aβ1-42 injection into the hippocampus. Cognitive functions were assessed using the Passive Avoidance (PA) and Morris Water Maze (MWM) tests. After behavioral experiments, hippocampal cytokine (tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β)) levels, as well as astrocyte and microglial activation, are evaluated. TQ treatment reversed memory impairements in the AD group. Hippocampal TNF-α and IL-1β levels were elevated in the AD and reduced in TQ-treated groups. Immunohistochemical analysis revealed that the increased reactivity of glial fibrillary acidic protein (GFAP) and ionized calcium-binding adapter molecule 1 (Iba1) in the AD group was significantly attenuated by TQ treatment. Both 10 mg/kg and 30 mg/kg doses of TQ administration improved cognitive performance, reduced neuroinflammation, and mitigated glial activation in an Aβ-induced AD rat model. These findings suggest that TQ may serve as a promising neuroprotective agent for AD. Further studies are required to elucidate its molecular mechanisms and therapeutic potential in clinical settings.
百里醌改善淀粉样蛋白诱导的阿尔茨海默病大鼠模型的认知障碍和神经炎症
阿尔茨海默病(AD)是一种进行性神经退行性疾病,以认知能力下降和记忆障碍为特征。淀粉样蛋白- β (Aβ)肽积累是阿尔茨海默病最重要的特征之一,可引起神经损伤和神经炎症。百里醌(TQ)是一种从黑草中提取的生物活性化合物,在以往的研究中显示出神经保护作用。本研究旨在评价TQ对a β1-42诱导的AD大鼠模型的改善作用。将雄性Wistar-Albino大鼠分为对照组、AD组、TQ-10 (10 mg/kg TQ)组和TQ-30 (30 mg/kg TQ)组。在海马注射Aβ1-42前7天和后10天口服TQ。采用被动回避(PA)和Morris水迷宫(MWM)测试评估认知功能。行为实验后,评估海马细胞因子(肿瘤坏死因子-α (TNF-α)和白细胞介素-1β (IL-1β))水平以及星形胶质细胞和小胶质细胞活化情况。TQ治疗逆转了AD组的记忆损伤。AD组海马TNF-α和IL-1β水平升高,tq组海马TNF-α和IL-1β水平降低。免疫组化分析显示,经TQ处理后,AD组神经胶质原纤维酸性蛋白(GFAP)和离子钙结合适配器分子1 (Iba1)的反应性升高明显减弱。在a β诱导的AD大鼠模型中,10 mg/kg和30 mg/kg剂量的TQ均能改善认知能力,减少神经炎症,并减轻神经胶质活化。这些发现提示TQ可能作为一种有前途的AD神经保护剂。需要进一步的研究来阐明其分子机制和临床治疗潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Brain Research
医学-神经科学
CiteScore
5.90
自引率
3.40%
发文量
268
审稿时长
47 days
期刊介绍:
An international multidisciplinary journal devoted to fundamental research in the brain sciences.
Brain Research publishes papers reporting interdisciplinary investigations of nervous system structure and function that are of general interest to the international community of neuroscientists. As is evident from the journals name, its scope is broad, ranging from cellular and molecular studies through systems neuroscience, cognition and disease. Invited reviews are also published; suggestions for and inquiries about potential reviews are welcomed.
With the appearance of the final issue of the 2011 subscription, Vol. 67/1-2 (24 June 2011), Brain Research Reviews has ceased publication as a distinct journal separate from Brain Research. Review articles accepted for Brain Research are now published in that journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


