The neurobehavioral impact of stress and the therapeutic role of valproic acid in a PTSD animal model
IF 2.6
4区 医学
Q3 NEUROSCIENCES
引用次数: 0
Abstract
This study examines the impact of stress on cognitive and behavioral functions and evaluates Valproic Acid (VPA) as a potential therapy in a mouse model of Post-Traumatic Stress Disorder (PTSD). Sixty-three male mice were assigned to control, PTSD, VPA, and PTSD + VPA groups, with behavioral assessments including anxiety, social interaction, memory, and depression-like tests. Histological analyses assessed neuronal integrity in the hippocampus, amygdala, cingulate cortex, and insula cortex. Stress significantly impaired cognitive performance, indicated by a lower Preference Index (PI%) in the novel object recognition task. Social interaction tests revealed decreased engagement in the PTSD group, reflecting social withdrawal. Behavioral assessments indicated increased immobility time in the tail suspension and forced swimming tests among PTSD mice, suggesting depression-like behaviors. In contrast, VPA treatment reduced immobility time, with the PTSD + VPA group showing improvements comparable to controls. Histological evaluations showed a higher percentage of degenerated cells in the hippocampus, amygdala, cingulate cortex, and insula cortex of the PTSD group versus controls. VPA administration significantly lowered the percentage of degenerated cells in the PTSD + VPA group, indicating neuroprotective effects, which were also noted in the hippocampus, amygdala, cingulate cortex, and insula cortex. These findings suggest VPA’s potential as a therapeutic agent for PTSD, warranting further pharmacological investigation.
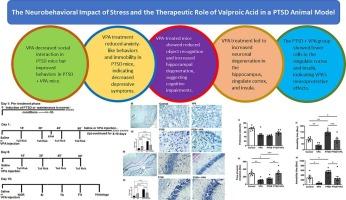
应激的神经行为影响和丙戊酸在创伤后应激障碍动物模型中的治疗作用
本研究探讨了应激对认知和行为功能的影响,并评估了丙戊酸(VPA)作为创伤后应激障碍(PTSD)小鼠模型的潜在治疗方法。63只雄性小鼠被分配到对照组、PTSD组、VPA组和PTSD + VPA组,进行行为评估,包括焦虑、社会互动、记忆和抑郁样测试。组织学分析评估了海马、杏仁核、扣带皮层和脑岛皮层的神经元完整性。压力显著损害认知表现,表现为在新物体识别任务中的偏好指数(PI%)较低。社交互动测试显示,PTSD组的参与度下降,反映出社交退缩。行为评估表明,PTSD小鼠在悬尾和强迫游泳测试中不动时间增加,表明有类似抑郁的行为。相比之下,VPA治疗减少了不动时间,PTSD + VPA组的改善与对照组相当。组织学评估显示,与对照组相比,PTSD组海马、杏仁核、扣带皮层和脑岛皮层中变性细胞的比例更高。在PTSD + VPA组中,VPA给药显著降低了退行性细胞的百分比,表明其具有神经保护作用,在海马、杏仁核、扣带皮层和岛皮质中也存在神经保护作用。这些发现表明VPA作为创伤后应激障碍治疗药物的潜力,值得进一步的药理学研究。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Brain Research
医学-神经科学
CiteScore
5.90
自引率
3.40%
发文量
268
审稿时长
47 days
期刊介绍:
An international multidisciplinary journal devoted to fundamental research in the brain sciences.
Brain Research publishes papers reporting interdisciplinary investigations of nervous system structure and function that are of general interest to the international community of neuroscientists. As is evident from the journals name, its scope is broad, ranging from cellular and molecular studies through systems neuroscience, cognition and disease. Invited reviews are also published; suggestions for and inquiries about potential reviews are welcomed.
With the appearance of the final issue of the 2011 subscription, Vol. 67/1-2 (24 June 2011), Brain Research Reviews has ceased publication as a distinct journal separate from Brain Research. Review articles accepted for Brain Research are now published in that journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


