Exogenous cargo-loaded extracellular vesicles as therapeutics: A review of pre-clinical studies
IF 4.9
3区 医学
Q1 PHARMACOLOGY & PHARMACY
Journal of Drug Delivery Science and Technology
Pub Date : 2025-08-31
DOI:10.1016/j.jddst.2025.107473
引用次数: 0
Abstract
Once cast aside and overlooked, extracellular vesicles (EVs) were long believed to be mere vesicles carrying cellular waste. Although identified four decades ago, only in recent years have EVs gained global recognition for their potential as innovative therapeutic and diagnostic agents. These natural lipid nanoparticles are capable of being exogenously loaded with a wide range of therapeutic cargo. Endogenous origin confers biocompatibility, while nanoscale size enables EVs to overcome the various biological barriers with ease, making them a promising drug delivery platform that can be harnessed for the treatment of several disease conditions. Moreover, the components present on the EV surface can be modified for enhanced loading to achieve a more precise targeted cargo delivery. This comprehensive review summarizes the current literature on exogenously cargo-loaded EVs, including their exogenous loading methods, targeting mechanisms, intracellular delivery, clinical translation, biodistribution, and in vivo visualization. The review also discusses preclinical studies utilizing cargo-loaded EVs for treating various disease conditions, while highlighting the commonly encountered clinical and regulatory hurdles in the successful translation of exogenous cargo-loaded EV-based therapeutics.
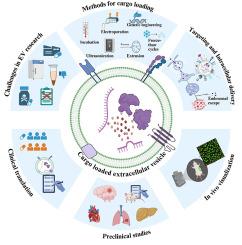
外源性载货细胞外囊泡作为治疗:临床前研究综述
一旦被抛弃和忽视,细胞外囊泡(EVs)长期以来被认为仅仅是携带细胞废物的囊泡。尽管早在40年前就被发现,但直到最近几年,电动汽车才因其作为创新治疗和诊断试剂的潜力而获得全球认可。这些天然脂质纳米颗粒能够外源性装载广泛的治疗货物。内源性来源赋予了生物相容性,而纳米尺度的尺寸使电动汽车能够轻松克服各种生物屏障,使其成为一种有前途的药物输送平台,可以用于治疗多种疾病。此外,电动汽车表面的组件可以进行修改,以增强负载,以实现更精确的目标货物交付。本文综述了外源性负载ev的相关文献,包括外源性负载方法、靶向机制、细胞内递送、临床翻译、生物分布和体内可视化。该综述还讨论了利用载货ev治疗各种疾病的临床前研究,同时强调了在成功翻译外源性载货ev治疗方法中常见的临床和监管障碍。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.00
自引率
8.00%
发文量
879
审稿时长
94 days
期刊介绍:
The Journal of Drug Delivery Science and Technology is an international journal devoted to drug delivery and pharmaceutical technology. The journal covers all innovative aspects of all pharmaceutical dosage forms and the most advanced research on controlled release, bioavailability and drug absorption, nanomedicines, gene delivery, tissue engineering, etc. Hot topics, related to manufacturing processes and quality control, are also welcomed.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


