Hydrated eutectic electrolyte enabling stable low-temperature zinc metal batteries with a tuned inner Helmholtz plane and a solid electrolyte interface
IF 14.9
1区 化学
Q1 Energy
引用次数: 0
Abstract
Aqueous zinc metal batteries (ZMBs) are promising for grid-scale energy storage, but their practical application is hindered by limited cycling life and inferior low-temperature performance, primarily due to Zn dendrite growth and parasitic reactions at the electrolyte-electrode interface. To address these challenges, we develop a new and cheap hydrated eutectic electrolyte (HEE) composed of ZnCl2, choline chloride (ChCl), and H2O, which can fundamentally tune desirable interface chemistries for dendrite-free and low-temperature ZMBs. The optimized HEE with a solvation structure of ZnCl3(ChCl)(H2O)2 shows a high conductivity of 15.98 mS cm−1 and excellent freeze resistance below −40 °C. It has been found that hydrogen bonding between ChCl and H2O effectively reduces water activity, while preferential adsorption of ChCl molecules at the inner Helmholtz plane promotes the formation of a protective solid electrolyte interphase (SEI) on Zn metal anodes, which greatly suppresses the dendrites and side reactions. Therefore, the HEE endows the as-fabricated Zn//Zn symmetric cells and Zn//polyaniline full batteries with superior electrochemical performance at −40 °C, such as a long cycling life of 870 h at 1 mA cm−2 and 1 mAh cm−2 and a high capacity of 75 mAh g−1 at 0.3 A g−1. The HEE reported here may pave a new way to construct high-performance ZMBs for specific low-temperature application scenarios.
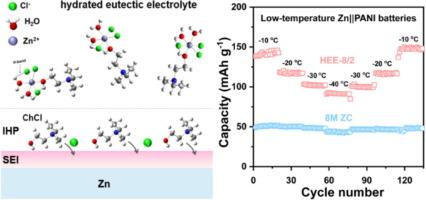
水合共晶电解质,使稳定的低温锌金属电池具有调谐的内部亥姆霍兹平面和固体电解质界面
水锌金属电池(zmb)在电网规模储能方面前景广阔,但其实际应用受到循环寿命有限和低温性能较差的阻碍,主要是由于锌枝晶生长和电解质-电极界面的寄生反应。为了解决这些挑战,我们开发了一种由ZnCl2,氯化胆碱(ChCl)和H2O组成的新型廉价水合共晶电解质(HEE),它可以从根本上调整无枝晶和低温zmb所需的界面化学。优化后的HEE具有ZnCl3(ChCl)(H2O)2的溶剂化结构,电导率为15.98 mS cm−1,在−40℃以下具有优异的抗冻性能。研究发现,ChCl与H2O之间的氢键可以有效地降低水的活度,而ChCl分子在内部亥姆霍兹平面上的优先吸附促进了Zn金属阳极上保护性固体电解质界面(SEI)的形成,极大地抑制了枝晶和副反应。因此,制备的Zn//Zn对称电池和Zn//聚苯胺全电池在- 40°C下具有优异的电化学性能,例如在1 mA cm - 2和1 mAh cm - 2下的长循环寿命为870 h,在0.3 a g - 1下的高容量为75 mAh g - 1。本文报道的HEE可能为构建适用于特定低温应用场景的高性能zmb铺平了新的道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


