Inactivation of LHAVglut2 neurons relieves stress-induced intestine inflammation by sympathetic nerve- intestinal epithelial cell Cxcl1 communication
IF 3.7
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Purpose
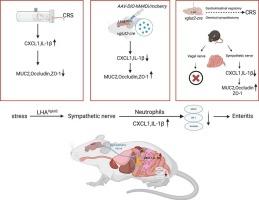
This study aimed to elucidate the role of lateral hypothalamic area (LHA) Vglut2 neurons in stress-induced intestinal inflammation and to investigate the underlying mechanisms involving neuro-immune interactions. Specifically, we hypothesized that LHA Vglut2 neuron activation exacerbates intestinal inflammation via sympathetic-driven IL-1β and Cxcl1 signaling.
Methods
Transgenic mice (Vglut2-cre) and wild-type controls were subjected to chronic restraint stress (CRS). Chemogenetic silencing of LHA Vglut2 neurons was achieved using hM4Di DREADD receptors. Techniques included qPCR, RNA sequencing, pseudorabies virus (PRV) retrograde tracing, immunofluorescence, and histopathology. Sympathetic ablation (6-OHDA) and vagotomy were performed to dissect neural pathways.
Results
CRS upregulated IL-1β and Cxcl1 in the gut, increased c-Fos expression in LHA neurons, and impaired intestinal barrier integrity (reduced ZO-1/Occludin, elevated MUC2). Silencing LHA Vglut2 neurons reversed these effects, reducing inflammation and restoring barrier proteins. RNA sequencing revealed IL-1β-Cxcl1 as a key pathway. Sympathetic ablation mirrored these improvements, while vagotomy showed no effect, indicating a predominant sympathetic-mediated mechanism.
Conclusion
LHA Vglut2 neurons drive stress-induced intestinal inflammation via sympathetic activation of the IL-1β-Cxcl1 axis. Targeting this hypothalamic-sympathetic circuit may offer therapeutic potential for stress-related gastrointestinal disorders.
LHAVglut2神经元失活可通过交感神经-肠上皮细胞Cxcl1通讯缓解应激性肠炎症
目的研究下丘脑外侧区(LHA) Vglut2神经元在应激性肠道炎症中的作用,并探讨其神经免疫相互作用的潜在机制。具体来说,我们假设LHA Vglut2神经元激活通过交感神经驱动的IL-1β和Cxcl1信号通路加剧肠道炎症。方法对转基因小鼠(Vglut2-cre)和野生型小鼠进行慢性抑制应激(CRS)。利用hM4Di DREADD受体实现LHA Vglut2神经元的化学发生沉默。技术包括qPCR, RNA测序,伪狂犬病毒(PRV)逆行示踪,免疫荧光和组织病理学。采用交感神经消融术(6-OHDA)和迷走神经切开术解剖神经通路。结果scrs上调肠道IL-1β和Cxcl1,增加LHA神经元中c-Fos的表达,损害肠屏障完整性(ZO-1/Occludin降低,MUC2升高)。沉默LHA Vglut2神经元逆转了这些作用,减少了炎症并恢复了屏障蛋白。RNA测序显示IL-1β-Cxcl1是关键通路。交感神经消融术反映了这些改善,而迷走神经切开术没有效果,表明交感神经介导的机制占主导地位。结论lha Vglut2神经元通过激活IL-1β-Cxcl1轴驱动应激性肠道炎症。以这种下丘脑-交感神经回路为目标,可能为应激相关的胃肠道疾病提供治疗潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cytokine
医学-免疫学
CiteScore
7.60
自引率
2.60%
发文量
262
审稿时长
48 days
期刊介绍:
The journal Cytokine has an open access mirror journal Cytokine: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
* Devoted exclusively to the study of the molecular biology, genetics, biochemistry, immunology, genome-wide association studies, pathobiology, diagnostic and clinical applications of all known interleukins, hematopoietic factors, growth factors, cytotoxins, interferons, new cytokines, and chemokines, Cytokine provides comprehensive coverage of cytokines and their mechanisms of actions, 12 times a year by publishing original high quality refereed scientific papers from prominent investigators in both the academic and industrial sectors.
We will publish 3 major types of manuscripts:
1) Original manuscripts describing research results.
2) Basic and clinical reviews describing cytokine actions and regulation.
3) Short commentaries/perspectives on recently published aspects of cytokines, pathogenesis and clinical results.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


