Opisthorchis viverrini Helminth Defense Molecule: Structural features, molecular interactions, and dual immunomodulatory roles
IF 2.5
3区 医学
Q2 PARASITOLOGY
引用次数: 0
Abstract
The liver fluke Opisthorchis viverrini causes chronic infections in humans and animals. This helminth is known to coexist with the bacterial microbiome in the host's bile duct, and their interaction potentially impacts the helminth’s pathogenicity. While most infected individuals remain asymptomatic, the mechanism of immune modulation remains unclear. The immunomodulatory protein Helminth Defense Molecule (HDM) has been characterized in other liver fluke species; however, its functional role exhibits distinct variations. This study aims to uncover the structural properties and biological function of HDM from O.viverrini (OvHDM). Using bioinformatics tools, we predicted the protein structure, analyzed its physicochemical properties, and potential molecular interactions. Furthermore, the structural and molecular interactions were experimentally confirmed by Circular Dichroism (CD) Spectroscopy. For functional analysis, RAW 264.7 macrophage cells were treated with Escherichia coli LPS pre-incubated with synthetic OvHDM peptides, followed by TNF and IL-6 levels quantification using ELISA. The predicted structure of OvHDM predominantly consists of α-helices configuration, resembling the antimicrobial peptide LL-37. It exhibits a high distribution of positive amino acids, strong stability under physiological conditions, and potential functional sites in both the N- and C-terminal domains. OvHDM demonstrated stronger interactions with LPS compared to the FhHDM-1 P2 as a control. The CD spectroscopy showed secondary structure dynamics of HDMs synthetic peptides upon LPS addition. Finally, synthetic OvHDM peptides were shown to both reduced LPS-induced cytokine production in vitro, and independently induced IL-6 production. These findings highlight the dual immunomodulatory activity of OvHDM, suggesting its role in fine-tuning the host immune environment during infection.
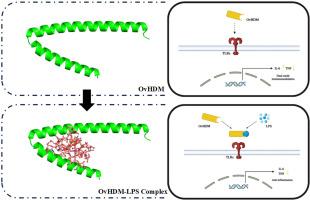
蠕虫防御分子:结构特征、分子相互作用和双重免疫调节作用
猪肝吸虫在人类和动物中引起慢性感染。已知这种蠕虫与宿主胆管中的细菌微生物群共存,它们的相互作用可能影响蠕虫的致病性。虽然大多数感染者仍然无症状,但免疫调节的机制尚不清楚。免疫调节蛋白蠕虫防御分子(HDM)已在其他肝吸虫物种中被表征;然而,其功能角色表现出明显的变化。本研究旨在揭示oviverrini (OvHDM) HDM的结构特性和生物学功能。利用生物信息学工具,我们预测了蛋白质结构,分析了其物理化学性质和潜在的分子相互作用。此外,通过圆二色光谱(CD)实验证实了结构和分子间的相互作用。为了进行功能分析,我们用大肠杆菌LPS预处理RAW 264.7巨噬细胞,并用合成的OvHDM肽孵育,然后用ELISA定量检测TNF和IL-6水平。OvHDM的预测结构主要由α-螺旋构型组成,类似于抗菌肽LL-37。它具有较高的正氨基酸分布,在生理条件下具有较强的稳定性,在N端和c端都具有潜在的功能位点。与对照的FhHDM-1 P2相比,OvHDM表现出与LPS更强的相互作用。CD光谱显示了添加LPS后HDMs合成肽的二级结构动态。最后,合成的OvHDM肽在体外被证明可以减少lps诱导的细胞因子的产生,并独立诱导IL-6的产生。这些发现突出了OvHDM的双重免疫调节活性,提示其在感染期间微调宿主免疫环境中的作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta tropica
医学-寄生虫学
CiteScore
5.40
自引率
11.10%
发文量
383
审稿时长
37 days
期刊介绍:
Acta Tropica, is an international journal on infectious diseases that covers public health sciences and biomedical research with particular emphasis on topics relevant to human and animal health in the tropics and the subtropics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


