Effect of hydration level on zinc electrolyte properties
IF 3.3
4区 材料科学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
We study zinc (Zn) plating and stripping in zinc cells using various compositions of hydrated zinc chloride (ZnCl₂·nH₂O) electrolyte (n = 10, n = 2.33, n = 1.5) at temperatures from −10 °C to 40 °C. The effect of deposition temperature on nuclei size, nucleation density, and growth of deposited Zn metal is studied by ex-situ microscopy investigations. Lowering the deposition temperature leads to smaller nuclei, higher nucleation density, and smoother zinc growth, forming a dendrite-free electrode surface. Compact and smooth zinc deposits contribute to long-term stability. There is no change in overpotential for the cell at −10 °C for more than 400 h. Long-term Zn deposition in Zn/Cu cells achieves an average coulombic efficiency (CE) of 99.2 % in the last 50 cycles using ZnCl₂·10H₂O electrolyte. The performance of electrolytes is evaluated in zinc-ion batteries with (zinc hexacyanoferrate) ZnHCF cathodes. The electrochemical studies show that the capacity of our zinc-ion battery in molten hydrated electrolytes increases from 17 mAh g−1 at −10 °C to 72 mAh g−1 at 40 °C, using n = 10 and n = 1.5 compositions of molten ZnCl₂ electrolyte. Increasing the temperature to 40 °C yields a high capacity and long-life zinc-ion battery. This research advances understanding of temperature-dependent molten hydrated electrolyte systems for zinc batteries.
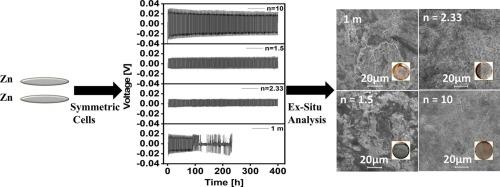
水化水平对锌电解质性能的影响
我们研究了锌电池中锌(Zn)的电镀和剥离,使用不同组成的水合氯化锌(ZnCl₂·nH₂O)电解质(n = 10, n = 2.33, n = 1.5),温度从- 10°C到40°C。采用非原位显微镜研究了沉积温度对锌金属核尺寸、成核密度和生长的影响。降低沉积温度可以使锌的核更小,成核密度更高,锌的生长更平滑,形成无枝晶的电极表面。致密光滑的锌矿有助于长期稳定。在−10°C下,电池的过电位在400小时内没有变化。在使用ZnCl₂·10H₂O电解质的情况下,锌/铜电池中的长期锌沉积在过去50个循环中达到了99.2%的平均库仑效率(CE)。在采用(六氰高铁锌)ZnHCF阴极的锌离子电池中,对电解质的性能进行了评价。电化学研究表明,在n = 10和n = 1.5的ZnCl 2溶液中,锌离子电池的容量从- 10°C时的17 mAh g−1增加到40°C时的72 mAh g−1。将温度提高到40°C可以得到高容量和长寿命的锌离子电池。这项研究促进了对锌电池温度依赖的熔融水合电解质系统的理解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Solid State Ionics
物理-物理:凝聚态物理
CiteScore
6.10
自引率
3.10%
发文量
152
审稿时长
58 days
期刊介绍:
This interdisciplinary journal is devoted to the physics, chemistry and materials science of diffusion, mass transport, and reactivity of solids. The major part of each issue is devoted to articles on:
(i) physics and chemistry of defects in solids;
(ii) reactions in and on solids, e.g. intercalation, corrosion, oxidation, sintering;
(iii) ion transport measurements, mechanisms and theory;
(iv) solid state electrochemistry;
(v) ionically-electronically mixed conducting solids.
Related technological applications are also included, provided their characteristics are interpreted in terms of the basic solid state properties.
Review papers and relevant symposium proceedings are welcome.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


