Amine-modified graphene oxide: Reaction mechanism with functional groups and enhanced CO2 adsorption-separation performance
IF 3.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
Amine-modified adsorbents exhibit remarkable potential for efficient CO2 capture. Nevertheless, the understanding of the reaction mechanism between amines and adsorbents, as well as the influence of polar groups on CO2 adsorption, remains rather limited. In this study, a series of amine-containing chemicals were used to modify graphene oxide (GO). This exploration revealed the reaction mechanism between amine groups and GO's oxygen functional groups and emphasized that GO with a higher amine density and stronger polar groups demonstrates enhanced CO2 adsorption and separation capabilities. Compared with pristine GO, urea-modified GO shows a significantly increased CO2 adsorption capacity (from 0.42 to 1.70 mmol/g) and CO2/N2 selectivity (from 3.0 to 28.0). After 10 adsorption-desorption cycles, its adsorption capacity decreased by only 7.56 %, confirming its good regenerative stability. Overall, the modified GO co-regulated by amine groups and polar groups prepared in this study is considered an effective CO2 adsorption/separation material, showing great potential for green and sustainable applications.
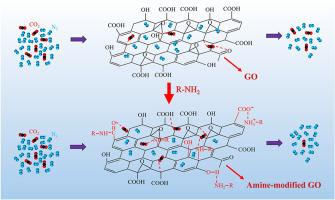
胺修饰氧化石墨烯:具有官能团的反应机理及增强CO2吸附分离性能
胺改性吸附剂在有效捕获二氧化碳方面表现出显著的潜力。然而,对于胺与吸附剂之间的反应机理以及极性基团对CO2吸附的影响的了解仍然相当有限。在这项研究中,使用了一系列含胺的化学物质来修饰氧化石墨烯(GO)。本研究揭示了胺基与氧化石墨烯氧官能团之间的反应机理,并强调了胺密度越高、极性基团越强的氧化石墨烯对CO2的吸附和分离能力越强。与原始氧化石墨烯相比,尿素修饰氧化石墨烯的CO2吸附量(从0.42提高到1.70 mmol/g)和CO2/N2选择性(从3.0提高到28.0)显著提高。经过10次吸附-解吸循环后,其吸附量仅下降7.56%,表明其具有良好的再生稳定性。综上所述,本研究制备的胺基和极性基共调控的改性氧化石墨烯被认为是一种有效的CO2吸附/分离材料,具有很大的绿色可持续应用潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Solid State Chemistry
化学-无机化学与核化学
CiteScore
6.00
自引率
9.10%
发文量
848
审稿时长
25 days
期刊介绍:
Covering major developments in the field of solid state chemistry and related areas such as ceramics and amorphous materials, the Journal of Solid State Chemistry features studies of chemical, structural, thermodynamic, electronic, magnetic, and optical properties and processes in solids.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


