Melittin-induced modulation of mitochondrial physiology: Beyond the antitumoral actions
IF 2.4
4区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Melittin (MEL), a cationic amphipathic peptide derived from bee venom, exhibits dual roles in mitochondrial physiology, with both cytoprotective and cytotoxic outcomes. This review synthesizes current findings on MEL-induced modulation of mitochondrial pathways in normal and cancer cells. Beyond its well-documented roles in apoptosis regulation, MEL influences mitochondrial function by altering membrane potential, regulating respiratory chain activity, and impacting ATP production. These effects are context-dependent and vary across normal and tumor cell models. MEL can attenuate mitochondrial dysfunction by preserving mitochondrial membrane integrity and reducing reactive oxygen species, while in cancer cells, it often promotes mitochondrial depolarization, cytochrome c release, and caspase activation, culminating in intrinsic apoptotic signaling. Importantly, MEL modulates the expression of key proteins such as BAX, BCL-2, and APAF-1, and interacts with signaling cascades including PI3K/Akt, NF-κB, MAPKs, and Nrf2/HO-1. Recent studies have demonstrated that MEL also regulates mitochondrial quality control mechanisms, including the stimulation of mitophagy through PINK1/Parkin and AMPK-related pathways. Moreover, MEL interacts directly with mitochondrial membranes and affects enzymes critical for energy metabolism, such as F1-ATPase, contributing to altered bioenergetic output. These actions suggest that MEL's mitochondrial effects extend beyond cell death regulation, encompassing broader control over metabolic activity, oxidative stress, and organelle maintenance. Future investigations should integrate redox biology, bioenergetics, and mitochondrial signaling to optimize MEL's therapeutic applications. Altogether, MEL represents a unique modulator of mitochondrial health, whose dual actions necessitate rigorous contextual evaluation for clinical translation.
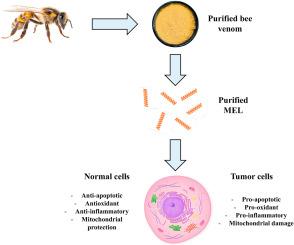
蜂毒素诱导的线粒体生理调节:超越抗肿瘤作用
蜂毒素(MEL)是一种从蜂毒中提取的阳离子两性肽,在线粒体生理学中表现出双重作用,具有细胞保护和细胞毒性。本文综述了mel诱导的正常细胞和癌细胞线粒体通路调节的最新研究结果。除了在细胞凋亡调节中的作用外,MEL还通过改变膜电位、调节呼吸链活性和影响ATP的产生来影响线粒体功能。这些影响是环境依赖的,并且在正常和肿瘤细胞模型中有所不同。MEL可以通过保持线粒体膜完整性和减少活性氧来减弱线粒体功能障碍,而在癌细胞中,它经常促进线粒体去极化、细胞色素c释放和caspase激活,最终产生固有的凋亡信号。重要的是,MEL调节关键蛋白如BAX、BCL-2和APAF-1的表达,并与信号级联包括PI3K/Akt、NF-κB、MAPKs和Nrf2/HO-1相互作用。最近的研究表明,MEL还调节线粒体质量控制机制,包括通过PINK1/Parkin和ampk相关途径刺激线粒体自噬。此外,MEL直接与线粒体膜相互作用,影响能量代谢的关键酶,如f1 - atp酶,导致生物能量输出的改变。这些作用表明,MEL的线粒体作用超出了细胞死亡调节,包括对代谢活动、氧化应激和细胞器维持的更广泛的控制。未来的研究应结合氧化还原生物学、生物能量学和线粒体信号来优化MEL的治疗应用。总之,MEL代表了线粒体健康的独特调节剂,其双重作用需要对临床翻译进行严格的上下文评估。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Toxicon
医学-毒理学
CiteScore
4.80
自引率
10.70%
发文量
358
审稿时长
68 days
期刊介绍:
Toxicon has an open access mirror Toxicon: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review. An introductory offer Toxicon: X - full waiver of the Open Access fee.
Toxicon''s "aims and scope" are to publish:
-articles containing the results of original research on problems related to toxins derived from animals, plants and microorganisms
-papers on novel findings related to the chemical, pharmacological, toxicological, and immunological properties of natural toxins
-molecular biological studies of toxins and other genes from poisonous and venomous organisms that advance understanding of the role or function of toxins
-clinical observations on poisoning and envenoming where a new therapeutic principle has been proposed or a decidedly superior clinical result has been obtained.
-material on the use of toxins as tools in studying biological processes and material on subjects related to venom and antivenom problems.
-articles on the translational application of toxins, for example as drugs and insecticides
-epidemiological studies on envenoming or poisoning, so long as they highlight a previously unrecognised medical problem or provide insight into the prevention or medical treatment of envenoming or poisoning. Retrospective surveys of hospital records, especially those lacking species identification, will not be considered for publication. Properly designed prospective community-based surveys are strongly encouraged.
-articles describing well-known activities of venoms, such as antibacterial, anticancer, and analgesic activities of arachnid venoms, without any attempt to define the mechanism of action or purify the active component, will not be considered for publication in Toxicon.
-review articles on problems related to toxinology.
To encourage the exchange of ideas, sections of the journal may be devoted to Short Communications, Letters to the Editor and activities of the affiliated societies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


