Acid-triggered nucleic acid release from gold nanoparticles via Schiff base linkages: In vitro validation of endosomal escape and gene silencing
IF 6
2区 医学
Q2 MATERIALS SCIENCE, BIOMATERIALS
Materials Science & Engineering C-Materials for Biological Applications
Pub Date : 2025-08-23
DOI:10.1016/j.bioadv.2025.214477
引用次数: 0
Abstract
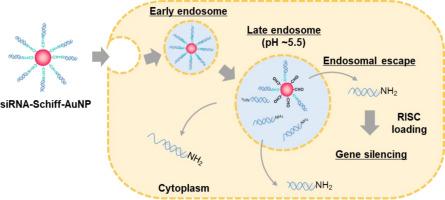
Gold nanoparticles with brush structures of nucleic acid drugs (Nuc–AuNPs) are prepared by mixing thiol-modified nucleic acid drugs and AuNPs due to the strong affinity of the Au–S bond. However, effectively regulating the intracellular kinetics of nucleic acids remains a challenge in achieving highly efficient nucleic-acid delivery. In this study, we designed new DNA-Schiff–AuNPs. The DNA release behaviors of these DNA-modified AuNPs were characterized under acidic conditions analogous to those in intracellular endosomes. Cy5-labeled DNA with an amine terminus (Cy5-DNA-NH2) reacted with 4-mercaptobenzaldehyde in dimethyl sulfoxide to form a Schiff base. The resulting Cy5-DNA-Schiff-SH was added to the AuNP dispersion system to prepare Cy5-DNA-Schiff–AuNPs. These nanoconjugates were characterized by fluorescence spectroscopy. In the appropriate buffer, the fluorescence intensity of AuNPs remained constant at pH 7.4, while exhibiting an approximately four-fold increase at pH 4.0 and 5.0. In addition, a drastic color change from red to blue was observed after 24 h, suggesting that DNA was released from AuNPs at a pH of 5.5. The DNA-Schiff–AuNPs were effectively incorporated into HeLa cells without any toxicity. Furthermore, siRNA–AuNPs incorporating Schiff base linkages demonstrated significantly enhanced suppression of epidermal growth factor receptor gene expression compared to conventional siRNA–AuNPs. These findings highlight the potential of Schiff base–linked Nuc–AuNPs as a promising platform for intracellular nucleic acid delivery.
酸触发的核酸通过希夫碱键从金纳米颗粒释放:内体逃逸和基因沉默的体外验证
利用Au-S键的强亲和力,将巯基修饰的核酸药物与AuNPs混合制备出具有核酸药物刷状结构的金纳米颗粒(Nuc-AuNPs)。然而,有效调节核酸的细胞内动力学仍然是实现高效核酸递送的一个挑战。在这项研究中,我们设计了新的DNA-Schiff-AuNPs。这些DNA修饰的AuNPs在酸性条件下的DNA释放行为与细胞内核体相似。带有胺端cy5标记的DNA (Cy5-DNA-NH2)在二甲亚砜中与4-巯基苯甲醛反应形成席夫碱。将得到的Cy5-DNA-Schiff-SH加入到AuNP分散体系中,制备Cy5-DNA-Schiff-AuNPs。利用荧光光谱对这些纳米缀合物进行了表征。在适当的缓冲液中,AuNPs的荧光强度在pH 7.4时保持不变,而在pH 4.0和5.0时表现出大约4倍的增加。此外,24 h后观察到颜色从红色到蓝色的剧烈变化,表明DNA在pH为5.5时从AuNPs中释放出来。DNA-Schiff-AuNPs被有效地整合到HeLa细胞中,没有任何毒性。此外,与传统的siRNA-AuNPs相比,结合希夫碱键的siRNA-AuNPs对表皮生长因子受体基因表达的抑制作用显著增强。这些发现突出了希夫碱基连接的Nuc-AuNPs作为细胞内核酸传递的有前途的平台的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
17.80
自引率
0.00%
发文量
501
审稿时长
27 days
期刊介绍:
Biomaterials Advances, previously known as Materials Science and Engineering: C-Materials for Biological Applications (P-ISSN: 0928-4931, E-ISSN: 1873-0191). Includes topics at the interface of the biomedical sciences and materials engineering. These topics include:
• Bioinspired and biomimetic materials for medical applications
• Materials of biological origin for medical applications
• Materials for "active" medical applications
• Self-assembling and self-healing materials for medical applications
• "Smart" (i.e., stimulus-response) materials for medical applications
• Ceramic, metallic, polymeric, and composite materials for medical applications
• Materials for in vivo sensing
• Materials for in vivo imaging
• Materials for delivery of pharmacologic agents and vaccines
• Novel approaches for characterizing and modeling materials for medical applications
Manuscripts on biological topics without a materials science component, or manuscripts on materials science without biological applications, will not be considered for publication in Materials Science and Engineering C. New submissions are first assessed for language, scope and originality (plagiarism check) and can be desk rejected before review if they need English language improvements, are out of scope or present excessive duplication with published sources.
Biomaterials Advances sits within Elsevier''s biomaterials science portfolio alongside Biomaterials, Materials Today Bio and Biomaterials and Biosystems. As part of the broader Materials Today family, Biomaterials Advances offers authors rigorous peer review, rapid decisions, and high visibility. We look forward to receiving your submissions!

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


