Boosting oxygen evolution reaction at hydrothermally synthesized V-MXene interface with Iron Pthalocynaine
IF 4.1
3区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
Oxygen evolution reaction (OER) is a pivotal electrochemical reaction process for many renewable energy technologies. Due to the sluggish OER kinetics, designing and fabricating efficient low-cost non-precious metal catalysts is one of the crucial but very challenging steps to develop electrochemically active and stable OER electrocatalyst. Conventionally, MXenes are prepared from hydrofluoric (HF) acid but the acute toxicity of HF acid impedes the wide utilization in energy related applications. Herein, V2C MXene is prepared through hydrothermal low level of danger HF free synthetic approach and synergistically coupled with Iron Phthalocyanine electrocatalyst. Moreover, the better OER efficiency of HF free hydrothermally synthesized MXene (with an overpotential of 373 mV) is described as compared to HF based MXene of OER overpotential of 384 mV at the current density of 10 mA/cm2. The inserted HT MX sheets within the matrix of FePc rods exhibited the desired crystallinity of hybrid. The XPS results suggest a synergistic chemical interaction between MX sheets and FePc molecules that modifies the electronic structure of the composite ensuring reduced charge transfer resistance. Consequently, the FePc:HT MX has shown appreciable OER electrocatalytic activity with an overpotential of 366 mV at a current density of 50 mA cm−2, Tafel slope of 5.36 mV dec−1 in 1 M KOH. Besides, the significant interaction between metallic centers and MXene support prevent detachment or agglomeration of active centers providing maximum interaction with the electrolytic ions, quick ionic OH− transportation, speedy and stable electron transfer thus ensure the long-term stability of composite during 50 h continuous operation of OER. In essence, this study features a facile approach for the hydrothermally synthesized MX sheets-based composites as advanced electrocatalysts for renewable energy applications.
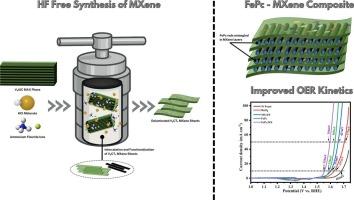
水热合成V-MXene界面与酞菁铁催化析氧反应
析氧反应(OER)是许多可再生能源技术的关键电化学反应过程。由于OER动力学缓慢,设计和制造高效低成本的非贵金属催化剂是开发电化学活性和稳定的OER电催化剂的关键但也是非常具有挑战性的步骤之一。传统上,MXenes是由氢氟酸制备的,但氢氟酸的急性毒性阻碍了其在能源相关领域的广泛应用。本文采用水热无危险HF合成方法制备了V2C MXene,并与酞菁铁电催化剂协同偶联。此外,在电流密度为10 mA/cm2时,与OER过电位为384 mV的HF基MXene相比,无HF水热合成的MXene(过电位为373 mV)的OER效率更高。在FePc棒基体中插入的HT - MX薄片显示出所需的杂化结晶度。XPS结果表明,MX薄片和FePc分子之间的协同化学相互作用改变了复合材料的电子结构,从而降低了电荷转移电阻。因此,FePc:HT MX在电流密度为50 mA cm−2时表现出明显的OER电催化活性,过电位为366 mV,在1 M KOH中Tafel斜率为5.36 mV dec−1。此外,金属中心与MXene载体之间的显著相互作用防止了活性中心的脱离或团聚,提供了与电解离子的最大相互作用,离子OH -的快速传递,快速稳定的电子转移,从而保证了复合材料在OER连续运行50 h时的长期稳定性。从本质上讲,这项研究的特点是水热合成MX片基复合材料作为可再生能源应用的先进电催化剂的简单方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
6.70%
发文量
912
审稿时长
2.4 months
期刊介绍:
The Journal of Electroanalytical Chemistry is the foremost international journal devoted to the interdisciplinary subject of electrochemistry in all its aspects, theoretical as well as applied.
Electrochemistry is a wide ranging area that is in a state of continuous evolution. Rather than compiling a long list of topics covered by the Journal, the editors would like to draw particular attention to the key issues of novelty, topicality and quality. Papers should present new and interesting electrochemical science in a way that is accessible to the reader. The presentation and discussion should be at a level that is consistent with the international status of the Journal. Reports describing the application of well-established techniques to problems that are essentially technical will not be accepted. Similarly, papers that report observations but fail to provide adequate interpretation will be rejected by the Editors. Papers dealing with technical electrochemistry should be submitted to other specialist journals unless the authors can show that their work provides substantially new insights into electrochemical processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


