Intranasal empagliflozin modulates synaptic plasticity in migraine: Insights into calcium signaling and epigenetic regulation
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
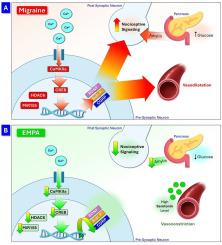
Migraine is a primary headache disorder without a definite pathophysiology or satisfactory managing strategies. Recently, migraine is primarily a disorder of brain plasticity coupled with altered Ca2+ dynamics regulating gene activity and protein expression. Further, epigenetics provided new insight into migraine pathogenesis and therapeutic response elucidation. Sodium-glucose co-transporter-2-inhibitors (e.g., empagliflozin (EMPA)), are a class of antihyperglycemic agents that can cross the blood-brain-barrier to maintain glucose homeostasis. EMPA possesses myriad pharmacological actions with potential beneficial effects for migraine management. Thus, the current study aimed at exploring EMPA efficacy and mechanisms for treating migraine headache, emphasizing its role in synaptic plasticity and epigenetic mechanisms.
Using an animal model of chronic migraine headache, the effect of oral (PO)/intranasal (IN) (brain-targeted)-EMPA versus Zolmitriptan (ZOL) on serum pain marker; Substance-P and migraine symptoms; pain, and photophobia were assessed biochemically and behaviorally. The influence on synaptic plasticity was evaluated by quantifying immunohistochemically stained synaptophysin in brain tissues and assessing calcium/calmodulin-activated-kinases (CaMKIIa)/ Camp-response-element-binding-protein (CREB)/calcitonin-gene-related-peptide (CGRP) or brain-derived-neurotrophic-factor (BDNF) pathways. Further, epigenetic modulation of migraine key genes was evaluated by determining HDAC6, CALCR, and MiR155–5p Moreover, serum glucose and amylin levels were appraised.
Both EMPA-PO/IN significantly decreased serum levels of substance-P and pain symptoms, increased brain serotonin levels, synaptophysin expression, and modulated the CaMKIIa/CREB/CGRP/or BDNF pathway. In addition, EMPA-IN, but not ZOL, down-regulated HDAC6, CALCR, and MiR155–5p expressions. Further, unlike EMPA-IN, ZOL, and EMPA-PO demonstrated significant hypoglycemic effects.
In conclusion, EMPA-IN shows potential as a novel therapeutic approach for managing migraine headaches by enhancing synaptic plasticity and modulating altered migraine-related epigenetic mechanisms.
恩格列净鼻内调节偏头痛的突触可塑性:钙信号和表观遗传调控的见解
偏头痛是一种原发性头痛疾病,没有明确的病理生理学或令人满意的治疗策略。最近,偏头痛主要是一种大脑可塑性紊乱,伴有调节基因活性和蛋白质表达的Ca2+动力学改变。此外,表观遗传学为偏头痛的发病机制和治疗反应的阐明提供了新的见解。钠-葡萄糖共转运蛋白-2抑制剂(如恩格列净(EMPA))是一类抗高血糖药物,可以穿过血脑屏障维持葡萄糖稳态。EMPA具有多种药理作用,对偏头痛的治疗具有潜在的有益作用。因此,本研究旨在探讨EMPA治疗偏头痛的疗效和机制,强调其在突触可塑性和表观遗传机制中的作用。采用慢性偏头痛动物模型,比较口服(PO)/鼻内(IN)(脑靶向)-EMPA与佐米曲坦(ZOL)对血清疼痛标志物的影响;p物质与偏头痛症状;对疼痛和畏光进行生化和行为学评估。通过定量免疫组织化学染色的脑组织突触素和评估钙/钙调素活化激酶(CaMKIIa)/ camp -反应元件结合蛋白(CREB)/降钙素基因相关肽(CGRP)或脑源性神经营养因子(BDNF)途径来评估对突触可塑性的影响。此外,通过检测HDAC6、CALCR和MiR155-5p来评估偏头痛关键基因的表观遗传调控。此外,还评估了血清葡萄糖和amylin水平。EMPA-PO/IN均可显著降低血清p物质水平和疼痛症状,增加脑5 -羟色胺水平和突触素表达,并调节CaMKIIa/CREB/CGRP/或BDNF通路。此外,EMPA-IN下调HDAC6、CALCR和MiR155-5p的表达,而ZOL不下调。此外,与EMPA-IN不同,ZOL和EMPA-PO表现出显著的降糖作用。总之,EMPA-IN通过增强突触可塑性和调节改变的偏头痛相关表观遗传机制,显示出作为一种治疗偏头痛的新方法的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


