Polystyrene nanoplastics reprogramed pulmonary metabolisms mediated by immune regulation of myeloid hypoxia-inducible factor 1α
IF 9.7
1区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
Exposure to nanoplastics (NPs), a pervasive environmental pollutant, presents potential health risks. Pulmonary exposure to NPs has been shown to disrupt both pulmonary metabolic status and immune homeostasis, leading to concerns about their impact on respiratory health and systemic well-being. However, the underlying linkage and mechanisms remain elusive. Following intratracheal instillation of polystyrene nanoplastics (PS-NPs) (5 μg/day for six weeks) in C57BL/6 mice, a combined approach of flow cytometry and metabolome analysis was applied to elucidate the interplay between metabolic and immune responses. Histopathological analysis indicated adverse lung effects from PS-NP exposure, characterized by immune cell infiltration and fibrosis. Flow cytometry analysis of lung immune cells further showed increased Ly6Clow monocyte and decreased neutrophil proportions. Metabolome analysis of the lungs of PS-NP-exposed mice revealed a metabolic shift with activated glycolysis and biosynthetic pathways. Such metabolic changes were consistent with hypoxia-inducible factor 1α (HIF-1α)-mediated upregulation of glycolysis, a metabolic phenotype commonly mimicking that of the activated myeloid cells during inflammation. Real-time quantitative PCR demonstrated glycolysis activation in the lungs and confirmed HIF-1α activation in myeloid cells using an in vitro RAW 264.7 macrophage model. To further investigate the contribution of HIF-1α in myeloid cells in lung metabolism, a myeloid cell-specific HIF-1α-deficient (Lyz2cre Hif1af/f) mouse model was employed. A shortened 2-week exposure experiment confirmed the indispensable role of HIF-1α in myeloid cells for metabolic modulation during PS-NP exposure. Spearman correlation analysis identified associations between immune cell populations and HIF-1α-related metabolites, suggesting potential crosstalk between HIF-1α-mediated metabolic alterations and immune changes induced by PS-NPs. Our study reveals the critical role of HIF-1α in myeloid cells in modulating lung metabolism and its potential crosstalk with the immune system, offering novel insights on the progression of NP-induced pulmonary toxicity.
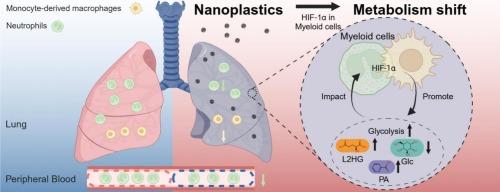
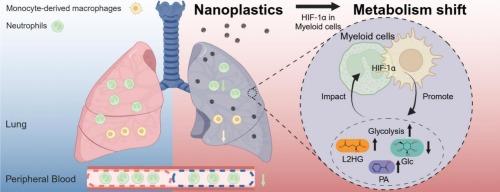
聚苯乙烯纳米塑料重编程髓系缺氧诱导因子1α免疫调节介导的肺代谢
纳米塑料(NPs)是一种普遍存在的环境污染物,暴露于纳米塑料中具有潜在的健康风险。肺部暴露于NPs已被证明会破坏肺部代谢状态和免疫稳态,导致人们担心它们对呼吸健康和全身健康的影响。然而,潜在的联系和机制仍然难以捉摸。在C57BL/6小鼠气管内灌注聚苯乙烯纳米塑料(PS-NPs)(5 μg/d,连续6周)后,采用流式细胞术和代谢组学分析相结合的方法来阐明代谢和免疫反应之间的相互作用。组织病理学分析表明,PS-NP暴露对肺有不良影响,其特征是免疫细胞浸润和纤维化。肺免疫细胞的流式细胞术分析进一步显示Ly6Clow单核细胞增加,中性粒细胞比例降低。ps - np暴露小鼠肺部的代谢组学分析显示,糖酵解和生物合成途径被激活,代谢发生变化。这种代谢变化与缺氧诱导因子1α (HIF-1α)介导的糖酵解上调一致,糖酵解是一种代谢表型,通常模拟炎症期间活化的髓样细胞。实时定量PCR证实肺中糖酵解激活,体外RAW 264.7巨噬细胞模型证实髓细胞中HIF-1α激活。为了进一步研究髓细胞HIF-1α在肺代谢中的作用,我们建立了髓细胞特异性HIF-1α-缺陷(Lyz2cre Hif1af/f)小鼠模型。缩短的2周暴露实验证实了HIF-1α在PS-NP暴露期间髓细胞代谢调节中不可或缺的作用。Spearman相关分析发现免疫细胞群与hif -1α相关代谢物之间存在关联,提示hif -1α介导的代谢改变与PS-NPs诱导的免疫变化之间存在潜在的串扰。我们的研究揭示了髓细胞中HIF-1α在调节肺代谢及其与免疫系统的潜在串扰中的关键作用,为np诱导的肺毒性的进展提供了新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Environment International
环境科学-环境科学
CiteScore
21.90
自引率
3.40%
发文量
734
审稿时长
2.8 months
期刊介绍:
Environmental Health publishes manuscripts focusing on critical aspects of environmental and occupational medicine, including studies in toxicology and epidemiology, to illuminate the human health implications of exposure to environmental hazards. The journal adopts an open-access model and practices open peer review.
It caters to scientists and practitioners across all environmental science domains, directly or indirectly impacting human health and well-being. With a commitment to enhancing the prevention of environmentally-related health risks, Environmental Health serves as a public health journal for the community and scientists engaged in matters of public health significance concerning the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


