Construction of chiral nitrogen stereocenters via enantioselective C–H activation
IF 19.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Enantioselective C–H activation has recently emerged as a versatile method for selectively constructing point, axial, and planar chirality. Out of the main strategies in asymmetric catalysis, desymmetrization, enantioselection, and classical kinetic resolutions have been demonstrated in C–H activation reactions. To the best of our knowledge, dynamic kinetic resolution (DKR) via enantioselective C(sp3)-H activation to set point chirality remains unrealized. While the rapid inversion of nitrogen stereocenters typically presents a challenge to their enantioselective synthesis, this phenomenon provides a unique opportunity to selectively access nitrogen stereocenters via a DKR given the appropriate system. Here, we report the DKR of nitrogen stereocenters via a Pd-catalyzed enantioselective C–H olefination yielding enantioenriched tribenzo[b,d,f]azepines and dibenzo[b,f]azepines, thus demonstrating the feasibility of constructing enantioenriched nitrogen stereocenters by harnessing nitrogen inversion. The chiral tribenzo[b,d,f]azepine products exhibit high circularly polarized luminescence (CPL) performance, representing a novel scaffold for exploration in this area.
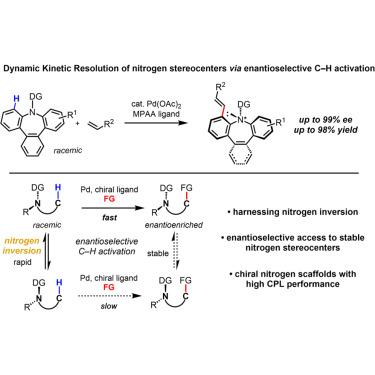
通过对映选择性碳氢活化构建手性氮立体中心
对映选择性碳氢化合物活化最近成为一种选择性地构建点、轴和平面手性的通用方法。在不对称催化的主要策略中,去对称化、对映体选择和经典动力学分解已经在碳氢活化反应中得到证实。据我们所知,通过对映选择性C(sp3)-H活化到设定点手性的动态动力学分辨率(DKR)仍未实现。虽然氮立体中心的快速反转通常对其对映选择性合成提出了挑战,但这种现象为通过给定适当系统的DKR选择性地获得氮立体中心提供了独特的机会。本研究报道了通过pd催化的对映选择性碳氢烯化反应生成富对映三苯[b,d,f]氮卓类化合物和二苯[b,f]氮卓类化合物,从而证明了利用氮转化构建富对映氮卓类化合物的可行性。手性三苯并[b,d,f]氮卓类化合物具有较高的圆偏振发光(CPL)性能,为该领域的研究提供了新的框架。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


