Divergent evolutionary strategies pre-empt tissue collision in gastrulation
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
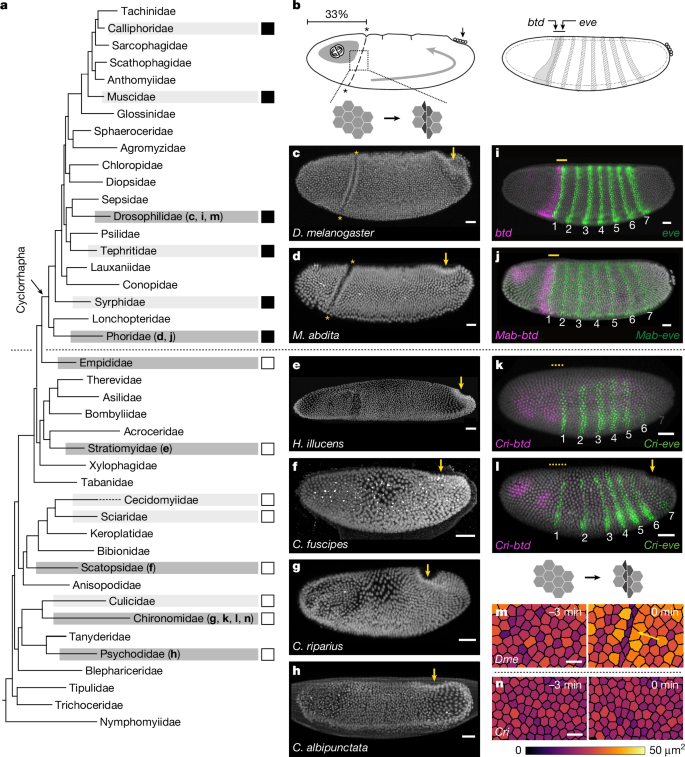
Metazoan development proceeds through a series of morphogenetic events that sculpt body plans and organ structures1,2. In the early embryo, these processes occur concurrently such that forces generated in neighbouring tissues can impose mechanical stresses on each other3–5, potentially disrupting development and consequently decreasing fitness. How organisms evolved mechanisms to mitigate inter-tissue mechanical conflicts remains unclear. Here, we combined phylogenetic survey, quantitative live imaging and functional mechanical perturbation to investigate mechanical stress management during gastrulation across the insect order of flies (Diptera). We identify two distinct cellular mechanisms that prevent tissue collision between the expanding head and trunk. In Cyclorrhapha, a monophyletic subgroup including Drosophila melanogaster, active out-of-plane deformation of a transient epithelial fold, called the cephalic furrow, acts as a mechanical sink to pre-empt head–trunk collision. Genetic and optogenetic ablation of the cephalic furrow leads to accumulation of compressive stress, tissue buckling at the head–trunk boundary and late-stage embryonic defects in the head and nervous system. By contrast, the non-cyclorrhaphan Chironomus riparius lacks cephalic furrow formation and instead undergoes widespread out-of-plane division that reduces the duration and spatial extent of head expansion. Re-orienting head mitosis from in-plane to out-of-plane in Drosophila partially suppresses tissue buckling, showing that it can function as an alternative mechanical sink. Our data suggest that mechanisms of mechanical stress management emerge and diverge in response to inter-tissue conflicts during early embryonic development. Flies have evolved two distinct strategies for managing mechanical stresses during embryogenesis: out-of-plane cell division in midges and transient out-of-plane tissue folding in fruit flies.

在原肠形成过程中,不同的进化策略可以预防组织碰撞
后生动物的发育通过一系列形态发生事件进行,这些事件塑造了身体计划和器官结构1,2。在早期胚胎中,这些过程同时发生,因此相邻组织产生的力可以相互施加机械应力3,4,5,潜在地破坏发育,从而降低适应性。生物体如何进化出机制来减轻组织间的机械冲突仍不清楚。在此,我们结合系统发育调查、定量实时成像和功能机械扰动研究了双翅目蝇原肠胚形成过程中的机械应力管理。我们确定了两种不同的细胞机制,防止组织碰撞之间的头部和躯干扩大。在环裂中,包括黑腹果蝇在内的单系亚群,被称为头沟的短暂上皮褶皱的主动面外变形,作为一个机械汇来先发制人的头部-躯干碰撞。遗传和光遗传消融头沟导致压缩应力积累,头干边界组织屈曲和头部和神经系统的晚期胚胎缺陷。相比之下,非环裂的滨水螯虾没有头沟形成,而是经历了广泛的面外分裂,减少了头部扩张的持续时间和空间范围。果蝇头部有丝分裂从平面内到平面外的重新定向部分抑制了组织屈曲,表明它可以作为一种替代的机械汇。我们的数据表明,机械应力管理机制在早期胚胎发育过程中响应组织间冲突而出现和分化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


