Unexpected diastereoselective chemistry on a 2D protein surface
IF 19.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Enantioselective enzymes feature structured catalytic sites positioned within binding pockets. In contrast, widely occurring flat protein surfaces, which have been suggested to have emerged early in ancestral protein evolution as solvent-exposed β sheets, appear unlikely to catalyze asymmetric reactions because they lack the necessary scaffold for interacting with substrates in three dimensions. At the near-flat, water-accessible surface within the α-hemolysin transmembrane pore, we now demonstrate remarkable diastereoselectivity in hemithioacetal formation between a cysteine side chain and a series of aldehyde substrates. After protein surface remodeling by mutagenesis, diastereomeric ratios of up to 95:5 (kinetic control) and 98:2 (thermodynamic control) were achieved with a range of aromatic aldehydes. Molecular dynamics simulations confirmed asymmetric interactions between adducts and nearby side chains in a two-dimensional plane. Our findings indicate that flat protein surfaces can scaffold stereoselective chemistry, thereby expanding the designable protein space for catalyst engineering and providing insight into the origin of selective enzymes.
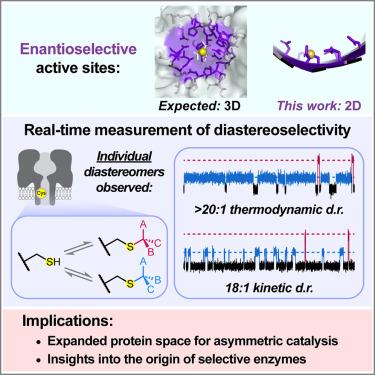
二维蛋白质表面意想不到的非对映选择性化学反应
对映选择性酶的特征是位于结合口袋内的结构催化位点。相比之下,广泛存在的扁平蛋白质表面,被认为是在祖先蛋白质进化的早期作为溶剂暴露的β片出现的,似乎不太可能催化不对称反应,因为它们缺乏与底物在三维上相互作用的必要支架。在α-溶血素跨膜孔内接近平坦的可水表面,我们现在证明了半胱氨酸侧链和一系列醛底物之间半缩醛形成的显着非对映选择性。通过诱变对蛋白质表面进行重塑后,一系列芳香族醛的非对构比达到95:5(动力学控制)和98:2(热力学控制)。分子动力学模拟证实了二维平面上加合物与侧链之间的不对称相互作用。我们的研究结果表明,平坦的蛋白质表面可以支撑立体选择性化学,从而扩大了催化剂工程的可设计蛋白质空间,并为选择性酶的起源提供了见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


