The HY5-NPR1 module governs light-dependent virulence of a plant bacterial pathogen
IF 18.7
1区 医学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
Light is essential for plant development, but its influence on pathogen virulence and immunity remains poorly understood. Here, we found that the Pseudomonas syringae DC3000 type III effector, AvrPtoB, exhibits virulence exclusively upon light exposure. This light-dependent regulation is controlled by the Arabidopsis transcription factor ELONGATED HYPOCOTYL 5 (HY5), a central regulator of photomorphogenesis. AvrPtoB targets HY5 in the nucleus, facilitating its ubiquitination and degradation. Genetic disruption of HY5 eliminates susceptibility to AvrPtoB and compromises plant immunity upon light exposure. HY5 enhances immunity by binding promoters of defense-related genes, activating their expression, and stabilizing the transcriptional coregulator NONEXPRESSOR OF PATHOGENESIS-RELATED (PR) GENES 1 (NPR1) by inhibiting its negative regulators NPR3/4. Both HY5-mediated immunity and light-dependent AvrPtoB virulence require NPR1. By contrast, during darkness, CONSTITUTIVELY PHOTOMORPHOGENIC 1 (COP1)-mediated HY5 degradation suppresses AvrPtoB virulence and HY5-enhanced immunity. These findings elucidate a mechanism in which light modulates bacterial virulence and plant immunity via an HY5-NPR1 module, advancing our understanding of light-pathogen-host interactions.
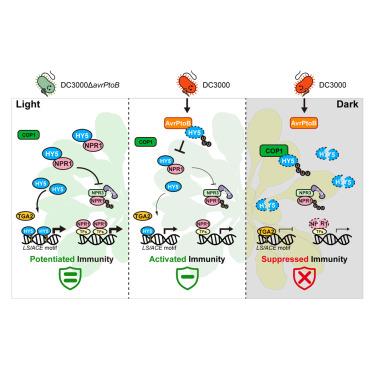
HY5-NPR1模块控制植物细菌病原体的光依赖性毒力
光对植物发育至关重要,但其对病原体毒力和免疫的影响尚不清楚。在这里,我们发现丁香假单胞菌DC3000 III型效应物AvrPtoB仅在光照下表现出毒力。这种光依赖性调节是由拟南芥转录因子伸长下胚轴5 (HY5)控制的,这是光形态发生的中心调节因子。AvrPtoB靶向细胞核中的HY5,促进其泛素化和降解。HY5的遗传破坏消除了对AvrPtoB的易感性,并损害了植物在光照下的免疫力。HY5通过结合防御相关基因的启动子,激活其表达,并通过抑制其负调控子NPR3/4来稳定转录共调控子NONEXPRESSOR of PATHOGENESIS-RELATED (PR) genes 1 (NPR1),从而增强免疫。hy5介导的免疫和光依赖性AvrPtoB毒力都需要NPR1。相反,在黑暗中,COP1介导的HY5降解抑制了AvrPtoB的毒力和HY5增强的免疫力。这些发现阐明了光通过HY5-NPR1模块调节细菌毒力和植物免疫的机制,促进了我们对光-病原体-宿主相互作用的理解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell host & microbe
生物-微生物学
CiteScore
45.10
自引率
1.70%
发文量
201
审稿时长
4-8 weeks
期刊介绍:
Cell Host & Microbe is a scientific journal that was launched in March 2007. The journal aims to provide a platform for scientists to exchange ideas and concepts related to the study of microbes and their interaction with host organisms at a molecular, cellular, and immune level. It publishes novel findings on a wide range of microorganisms including bacteria, fungi, parasites, and viruses. The journal focuses on the interface between the microbe and its host, whether the host is a vertebrate, invertebrate, or plant, and whether the microbe is pathogenic, non-pathogenic, or commensal. The integrated study of microbes and their interactions with each other, their host, and the cellular environment they inhabit is a unifying theme of the journal. The published work in Cell Host & Microbe is expected to be of exceptional significance within its field and also of interest to researchers in other areas. In addition to primary research articles, the journal features expert analysis, commentary, and reviews on current topics of interest in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


