Structural insights into the vitamin K-dependent γ-carboxylation of osteocalcin
IF 25.9
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
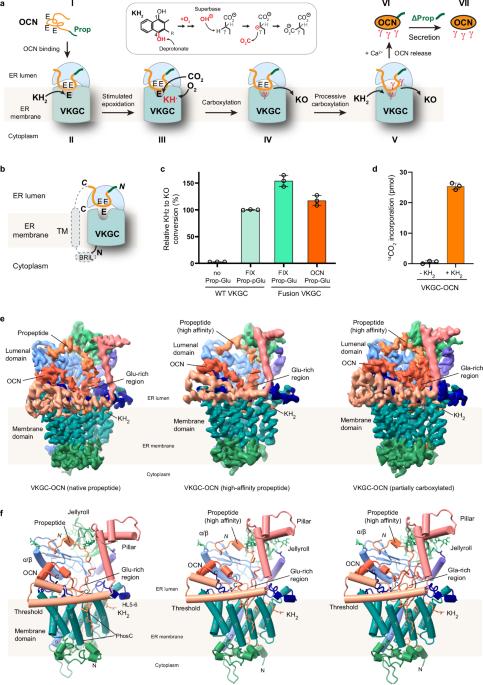
The γ-carboxylation state of osteocalcin determines its essential functions in bone mineralization or systemic metabolism and serves as a prominent biomarker for bone health and vitamin K nutrition. This post-translational modification of glutamate residues is catalyzed by the membrane-embedded vitamin K-dependent γ-carboxylase (VKGC), which typically recognizes protein substrates through their tightly bound propeptide that triggers γ-carboxylation. However, the osteocalcin propeptide exhibits negligible affinity for VKGC. To understand the underlying molecular mechanism, we determined the cryo-electron microscopy structures of VKGC with osteocalcin carrying a native propeptide or a high-affinity variant at different carboxylation states. The structures reveal a large chamber in VKGC that maintains uncarboxylated and partially carboxylated osteocalcin in partially unfolded conformations, allowing their glutamate-rich region and C-terminal helices to engage with VKGC at multiple sites. Binding of this mature region together with the low-affinity propeptide effectively stimulates VKGC activity, similar to high-affinity propeptides that differ only in closely fitting interactions. However, the low-affinity propeptide renders osteocalcin prone to undercarboxylation at low vitamin K levels, thereby serving as a discernible biomarker. Overall, our studies reveal the unique interaction of osteocalcin with VKGC and provide a framework for designing therapeutic strategies targeting osteocalcin-related bone and metabolic disorders.
对维生素k依赖性骨钙素γ-羧化的结构见解
骨钙素的γ-羧化状态决定了其在骨矿化或全身代谢中的基本功能,是骨骼健康和维生素K营养的重要生物标志物。这种谷氨酸残基的翻译后修饰是由膜嵌入的维生素k依赖性γ-羧化酶(VKGC)催化的,该酶通常通过紧密结合的前肽来识别蛋白质底物,从而触发γ-羧化。然而,骨钙素前肽对VKGC的亲和力可以忽略不计。为了了解潜在的分子机制,我们测定了骨钙素携带天然前肽或高亲和力变体在不同羧化状态下的VKGC的冷冻电镜结构。这些结构揭示了VKGC中的一个大腔室,该腔室将未羧化和部分羧化的骨钙素维持在部分展开的构象中,允许其富含谷氨酸的区域和c端螺旋在多个位点与VKGC结合。这一成熟区域与低亲和力前肽结合可有效刺激VKGC活性,类似于高亲和力前肽,其区别仅在于紧密配合的相互作用。然而,低亲和力的前肽使骨钙素在低维生素K水平下易于羧化,从而作为可识别的生物标志物。总之,我们的研究揭示了骨钙素与VKGC的独特相互作用,并为设计针对骨钙素相关骨和代谢紊乱的治疗策略提供了框架。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Research
生物-细胞生物学
CiteScore
53.90
自引率
0.70%
发文量
2420
审稿时长
2.3 months
期刊介绍:
Cell Research (CR) is an international journal published by Springer Nature in partnership with the Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences (CAS). It focuses on publishing original research articles and reviews in various areas of life sciences, particularly those related to molecular and cell biology. The journal covers a broad range of topics including cell growth, differentiation, and apoptosis; signal transduction; stem cell biology and development; chromatin, epigenetics, and transcription; RNA biology; structural and molecular biology; cancer biology and metabolism; immunity and molecular pathogenesis; molecular and cellular neuroscience; plant molecular and cell biology; and omics, system biology, and synthetic biology. CR is recognized as China's best international journal in life sciences and is part of Springer Nature's prestigious family of Molecular Cell Biology journals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


