A combined enteric neuron-gastric tumor organoid reveals metabolic vulnerabilities in gastric cancer
IF 20.4
1区 医学
Q1 CELL & TISSUE ENGINEERING
引用次数: 0
Abstract
The discrepancy between organoid and immortalized cell line cultures for cancer target discovery remains unclear. Here, our multi-tiered clustered regularly interspaced short palindromic repeats (CRISPR) screens reveal in vivo-relevant metabolic dependencies and synthetic lethal pairs that can be uncovered with tumor organoids but not cell lines or even three-dimensional (3D) spheroids. These screens identify lanosterol synthase and acetyl-coenzyme A (CoA) carboxylase inhibitors as effective treatments that impede xenografted tumor growth in mice. These lipid metabolic inhibitors exhibit nanomolar half-maximal inhibitory concentration (IC50) values across diverse human gastric cancer organoids resistant to first-line treatments. Mechanistically, gastric cancer organoids and in vivo tumors exhibit lipid metabolic adaptations not seen in two-dimensional (2D) in vitro cultures. Additionally, enteric neurons modulate lipid metabolism in tumor organoids, altering drug sensitivity by up to two orders of magnitude. A neuron-cocultured CRISPR screen further reveals that acetyl-CoA carboxylase expression determines lanosterol synthase inhibitor efficacy. These findings highlight the critical roles of organoid environment and neuronal interaction in cancer lipid reliance.
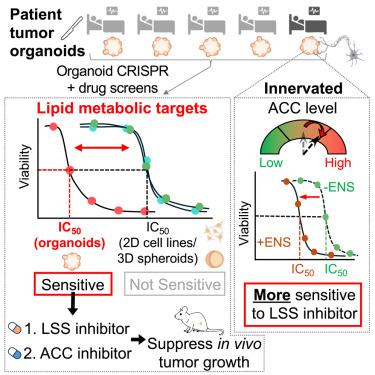
一个联合肠神经-胃肿瘤类器官揭示了胃癌的代谢脆弱性
类器官和永生化细胞系培养在发现癌症靶点方面的差异尚不清楚。在这里,我们的多层集群规则间隔短回复性重复序列(CRISPR)筛选揭示了体内相关的代谢依赖性和合成致死对,这些对可以在肿瘤类器官中发现,但不能在细胞系甚至三维(3D)球体中发现。这些筛选确定羊毛甾醇合成酶和乙酰辅酶A (CoA)羧化酶抑制剂是抑制小鼠异种移植肿瘤生长的有效治疗方法。这些脂质代谢抑制剂在不同的人胃癌类器官中表现出纳摩尔半最大抑制浓度(IC50)值,对一线治疗有耐药性。从机制上讲,胃癌类器官和体内肿瘤表现出在二维(2D)体外培养中未见的脂质代谢适应。此外,肠道神经元调节肿瘤类器官的脂质代谢,改变药物敏感性高达两个数量级。神经元共培养的CRISPR筛选进一步揭示了乙酰辅酶A羧化酶的表达决定了羊毛甾醇合成酶抑制剂的效果。这些发现强调了类器官环境和神经元相互作用在肿瘤脂质依赖中的关键作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell stem cell
生物-细胞生物学
CiteScore
37.10
自引率
2.50%
发文量
151
审稿时长
42 days
期刊介绍:
Cell Stem Cell is a comprehensive journal covering the entire spectrum of stem cell biology. It encompasses various topics, including embryonic stem cells, pluripotency, germline stem cells, tissue-specific stem cells, differentiation, epigenetics, genomics, cancer stem cells, stem cell niches, disease models, nuclear transfer technology, bioengineering, drug discovery, in vivo imaging, therapeutic applications, regenerative medicine, clinical insights, research policies, ethical considerations, and technical innovations. The journal welcomes studies from any model system providing insights into stem cell biology, with a focus on human stem cells. It publishes research reports of significant importance, along with review and analysis articles covering diverse aspects of stem cell research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


