An Escherichia coli Nissle 1917-based live therapeutics platform with integrated phage resistance and programmable temperature sensitivity
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Live bacterial therapeutics (LBT) represent a transformative modality for managing refractory chronic diseases. However, the absence of optimized microbial chassis systems is a significant barrier to clinical translation. To bridge this gap, we engineered Escherichia coli Nissle 1917 (EcN) into a versatile platform that meets the requirements for strain development and clinical application. First, to achieve precise transcriptional control, a constitutive promoter toolbox was constructed by hybridizing synthetic core promoters with engineered ribosome-binding sites. This library exhibited a 50.5-fold range in expression, enabling inducer-free fine-tuning of heterologous gene expression. Next, to ensure stable and antibiotic-free genetic maintenance, we repurposed the native plasmids pMUT1 and pMUT2 of EcN as modular shuttle vectors. This system was achieved through locus-specific integration, plasmid size minimization, and copy number amplification. The resulting plasmids, pMcol1 and pMrsf2, equipped with the high-strength hybrid promoter PJ23111-B0035, exhibited a 74- and 130-fold increase in reporter protein expression, respectively, compared to the pMUT1 and pMUT2 with the benchmark PUTR4-driven system. Both plasmids had a performance similar to that of the commercial pET-28a(+) system, demonstrating their high potential for diagnosis and treatment using EcN strains. To enhance bacterial persistence in the intestine, we genomically integrated a phosphorothioate-based defense module, which increased bacterial survival by 2.7-fold. This integration provides EcN with a defense mechanism against phages, thereby improving its stability and efficacy as a therapeutic agent. We also designed a temperature-sensitive kill switch circuit to induce bacterial elimination in the feces. This optimized EcN platform addresses challenges related to genetic stability, enteric survival, and biocontainment. Its therapeutic performance was validated in a murine colitis model, where it reduced histological inflammation, evidenced by intact crypt architecture and low immune cell infiltration. These findings underscore the high potential of this EcN platform for clinical translation and LBT applications.

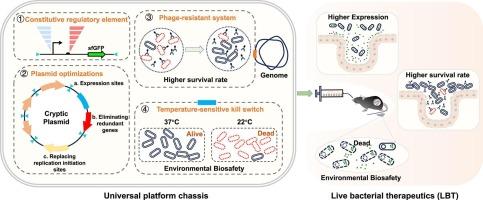
基于大肠杆菌Nissle 1917的活体治疗平台,集成了噬菌体抗性和可编程的温度敏感性
活细菌疗法(LBT)代表了治疗难治性慢性疾病的一种变革性模式。然而,缺乏优化的微生物底盘系统是临床转化的一个重大障碍。为了弥补这一差距,我们设计了大肠杆菌尼塞尔1917 (EcN)成为一个多功能平台,满足菌株开发和临床应用的要求。首先,为了实现精确的转录控制,通过将合成核心启动子与工程核糖体结合位点杂交构建了一个组成启动子工具箱。该文库具有50.5倍的表达范围,可以对外源基因的表达进行无诱导剂的微调。接下来,为了确保稳定和无抗生素的遗传维持,我们重新利用EcN的天然质粒pMUT1和pMUT2作为模块化穿梭载体。该系统是通过基因座特异性整合、质粒大小最小化和拷贝数扩增实现的。与putr4驱动的pMUT1和pMUT2相比,携带高强度杂交启动子PJ23111-B0035的质粒pMcol1和pMrsf2的报告蛋白表达分别增加了74倍和130倍。这两种质粒的性能与商用pET-28a(+)系统相似,表明它们在EcN菌株的诊断和治疗方面具有很高的潜力。为了增强细菌在肠道中的持久性,我们基因组整合了一个基于硫代酸的防御模块,使细菌存活率提高了2.7倍。这种整合为EcN提供了针对噬菌体的防御机制,从而提高了其作为治疗剂的稳定性和疗效。我们还设计了一个温度敏感的死亡开关电路,以诱导粪便中的细菌消除。这个优化的EcN平台解决了与遗传稳定性、肠道存活和生物控制相关的挑战。它的治疗效果在小鼠结肠炎模型中得到了验证,在小鼠结肠炎模型中,它减少了组织学炎症,证明了完整的隐窝结构和低免疫细胞浸润。这些发现强调了该EcN平台在临床翻译和LBT应用方面的巨大潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


