Simultaneous STING and lymphotoxin-β receptor activation induces B cell responses in tertiary lymphoid structures to potentiate antitumor immunity
IF 27.6
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
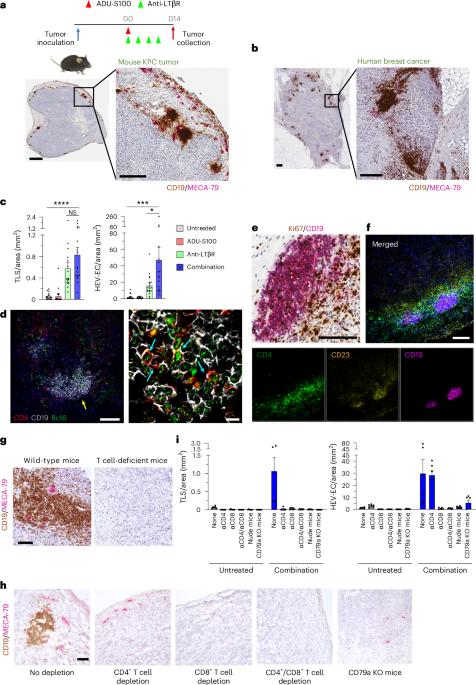
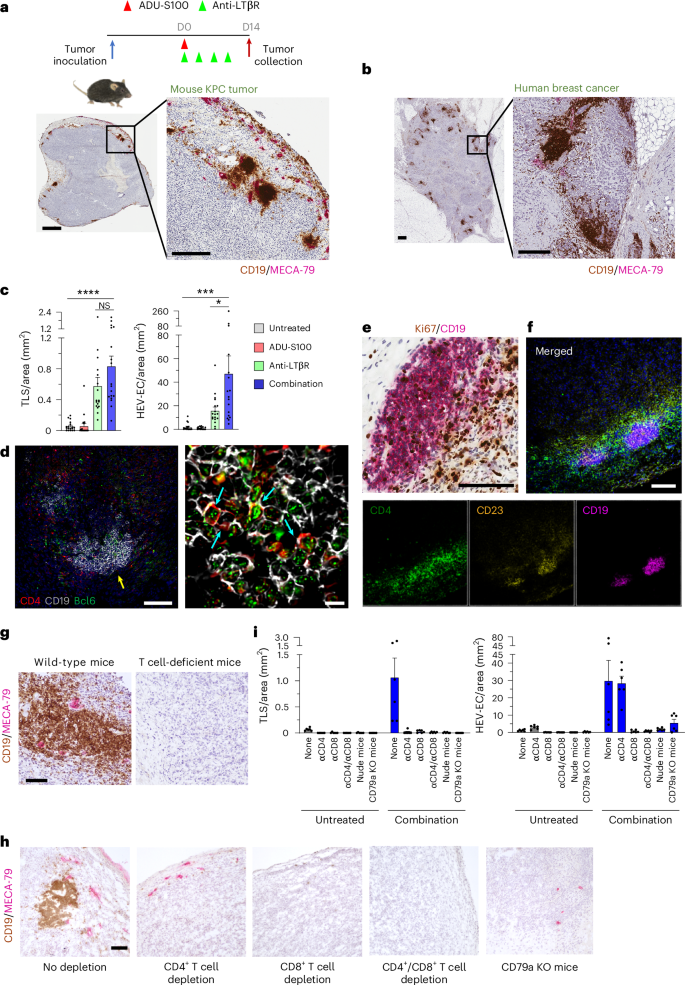
B cell-rich tertiary lymphoid structures (TLS) are associated with favorable prognosis and positive response to immunotherapy in cancer. Here we show that simultaneous activation of innate immune effectors, STING and lymphotoxin-β receptor (LTβR), results in CD8+ T cell-dependent tumor suppression while inducing high endothelial venule development and germinal center-like B cell responses in tumors to generate functional TLS in a T cell-dependent manner. In a neoadjuvant setting, activation of STING and LTβR by their agonists effectively immunized mice against tumor recurrence, leading to long-term survival. STING activation alone was insufficient for inducing B cell-containing TLS or eliciting long-term therapeutic effects. However, when combined with LTβR activation, it improved the fitness of TLS with B cell expansion and maturation to IgG-producing long-lived plasma cells and memory cells, increased CD4+ T cell recruitment and memory CD8+ T cell expansion, and shifted the TH2/TH17 balance, resulting in the potentiation of humoral and cellular immunity against tumors. These findings suggest a therapeutic approach of simultaneously activating STING and lymphotoxin pathways. Sawada et al. show simultaneous activation of the STING and lymphotoxin beta receptor signaling induces B cell-activating germinal center responses within tumor environment and enhances antitumor responses.
STING和淋巴毒素β受体同时激活可诱导三级淋巴结构中的B细胞反应,从而增强抗肿瘤免疫
富含B细胞的三级淋巴结构(TLS)与癌症患者良好的预后和对免疫治疗的积极反应有关。本研究表明,同时激活先天免疫效应物STING和淋巴毒素β受体(LTβR),导致CD8+ T细胞依赖性肿瘤抑制,同时诱导肿瘤中内皮小静脉的高度发育和生发中心样B细胞反应,以T细胞依赖性的方式产生功能性TLS。在新辅助治疗中,STING和LTβR的激动剂激活可以有效地免疫小鼠,使其免于肿瘤复发,从而获得长期生存。单独激活STING不足以诱导含B细胞的TLS或引起长期的治疗效果。然而,当与LTβR激活联合使用时,它提高了具有B细胞扩增和成熟的TLS对产生igg的长寿浆细胞和记忆细胞的适应性,增加了CD4+ T细胞募集和记忆CD8+ T细胞扩增,并改变了TH2/TH17平衡,从而增强了对肿瘤的体液和细胞免疫。这些发现提示了一种同时激活STING和淋巴毒素通路的治疗方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


