Genome-wide CRISPR screen identifies Menin and SUZ12 as regulators of human developmental timing
IF 19.1
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
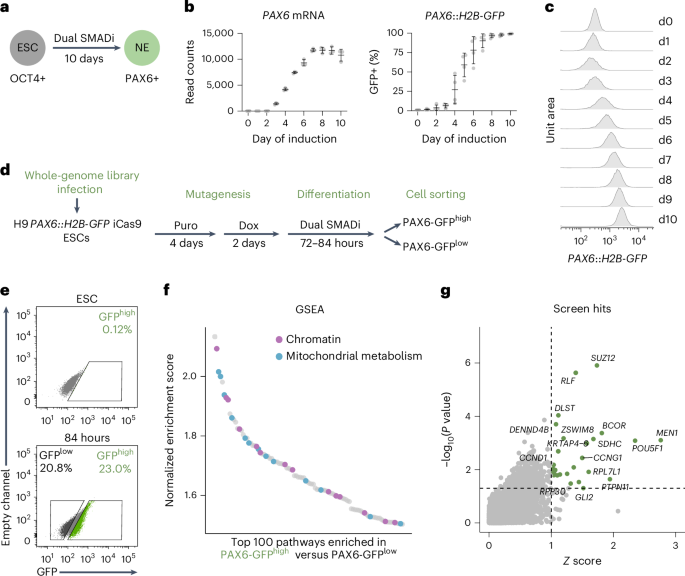
Embryonic development follows a conserved sequence of events across species, yet the pace of development is highly variable and particularly slow in humans. Species-specific developmental timing is largely recapitulated in stem cell models, suggesting a cell-intrinsic clock. Here we use directed differentiation of human embryonic stem cells into neuroectoderm to perform a whole-genome CRISPR-Cas9 knockout screen and show that the epigenetic factors Menin and SUZ12 modulate the speed of PAX6 expression during neural differentiation. Genetic and pharmacological loss-of-function of Menin or SUZ12 accelerate cell fate acquisition by shifting the balance of H3K4me3 and H3K27me3 at bivalent promoters, thereby priming key developmental genes for faster activation upon differentiation. We further reveal a synergistic interaction of Menin and SUZ12 in modulating differentiation speed. The acceleration effects were observed in definitive endoderm, cardiomyocyte and neuronal differentiation paradigms, pointing to chromatin bivalency as a general driver of timing across germ layers and developmental stages. Xu et al. perform a whole-genome CRISPR-Cas9 knockout screen using directed differentiation systems. They find that MEN1 and SUZ12 modulate human developmental timing by epigenetically controlling bivalent promoters of developmental genes.

全基因组CRISPR筛选发现Menin和SUZ12是人类发育时间的调节因子
不同物种的胚胎发育遵循一个保守的序列,但发育的速度是高度可变的,在人类中尤其缓慢。在干细胞模型中,物种特异性的发育时间在很大程度上得到了再现,这表明细胞具有内在的时钟。在这里,我们使用人类胚胎干细胞定向分化为神经外胚层进行全基因组CRISPR-Cas9敲除筛选,并显示表观遗传因子Menin和SUZ12调节神经分化过程中PAX6的表达速度。Menin或SUZ12的遗传和药理学功能丧失通过改变H3K4me3和H3K27me3在二价启动子上的平衡来加速细胞命运的获得,从而启动关键的发育基因,在分化时更快地激活。我们进一步揭示了Menin和SUZ12在调节分化速度方面的协同作用。在确定的内胚层、心肌细胞和神经元分化范式中观察到加速效应,指出染色质二价是跨胚层和发育阶段时间的一般驱动因素。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


