Gut microbiota dysbiosis amplifies thiram hepatotoxicity via a mitochondrial-autophagy-apoptosis nexus orchestrated by the gut-liver axis
IF 3.7
2区 生物学
Q2 CELL BIOLOGY
引用次数: 0
Abstract
Thiram, an environmentally persistent pesticide, poses significant hepatotoxic risks through oral exposure. However, the mechanisms linking gut dysbiosis to hepatic cell death remain unclear. Using a 5-week thiram exposure mouse model, we demonstrate that thiram-induced gut microbiota dysbiosis amplifies hepatotoxicity by disrupting the mitochondrial-autophagy-apoptosis axis via the gut-liver axis. We found that oral thiram exposure severely compromises intestinal barrier integrity, evidenced by colonic muscular layer thinning, goblet cell depletion, and downregulation of tight junction proteins (ZO-1, Occludin). Simultaneously, thiram induces dysbiosis characterized by an elevated Firmicutes/Bacteroidetes (F/B) ratio and altered abundances of Muribaculum intestinale, Bacteroides caecimuris, Lactobacillus johnsonii. These gut-derived perturbations triggered hepatic mitochondrial dynamics imbalance, driving aberrant de novo fatty acid synthesis and β-oxidation. Crucially, thiram disrupted autophagic flux and activated the mitochondrial apoptosis pathway, establishing a self-amplifying cycle of metabolic dysfunction and cellular demise. Our findings reveal that gut-mediated disruption of fatty acid metabolism orchestrates the collapse of crosstalk between mitochondria, autophagy, and apoptosis, ultimately culminating in hepatotoxicity. This work establishes the gut-liver axis as a central regulator of environmentally triggered apoptotic cell death.
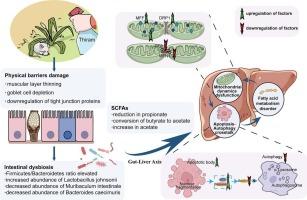
肠道菌群失调通过肠-肝轴协调的线粒体-自噬-凋亡关系放大肝毒性
噻蓝是一种环境持久性农药,通过口服接触会造成严重的肝毒性风险。然而,将肠道生态失调与肝细胞死亡联系起来的机制仍不清楚。通过5周的thiram暴露小鼠模型,我们证明thiram诱导的肠道微生物群失调通过肠-肝轴破坏线粒体-自噬-凋亡轴,从而放大肝毒性。我们发现口服硫丹暴露严重损害肠屏障的完整性,结肠肌肉层变薄、杯状细胞耗损和紧密连接蛋白(ZO-1, Occludin)下调就是证据。同时,thiram诱导菌群失调,其特征是厚壁菌门/拟杆菌门(F/B)比升高,肠道Muribaculum ininale, caecimuris Bacteroides, johnsonii乳杆菌丰度改变。这些肠道来源的扰动引发肝脏线粒体动力学失衡,驱动异常的脂肪酸合成和β氧化。至关重要的是,thiram破坏了自噬通量,激活了线粒体凋亡途径,建立了代谢功能障碍和细胞死亡的自我放大循环。我们的研究结果表明,肠道介导的脂肪酸代谢破坏协调了线粒体、自噬和细胞凋亡之间的串扰崩溃,最终导致肝毒性。这项工作建立了肠-肝轴作为环境引发的凋亡细胞死亡的中心调节器。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cellular signalling
生物-细胞生物学
CiteScore
8.40
自引率
0.00%
发文量
250
审稿时长
27 days
期刊介绍:
Cellular Signalling publishes original research describing fundamental and clinical findings on the mechanisms, actions and structural components of cellular signalling systems in vitro and in vivo.
Cellular Signalling aims at full length research papers defining signalling systems ranging from microorganisms to cells, tissues and higher organisms.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


