Phosphine-mediated deoxygenative synthesis of amides from carboxylic acids and N-chloro compounds
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A strategy for amide synthesis through the deoxygenation of carboxylic acids with N-chloro compounds using a phosphine-mediated P(V) platform is reported. This method enables the efficient conversion of various carboxylic acids, including primary, secondary, and bioactive molecules, as well as amino acids, into amides under mild conditions. The scalability of the reaction highlights its practical applicability for large-scale amide production. This approach offers a new perspective on activation reagents, focusing on aniline activation rather than conventional carboxylic acid activation.
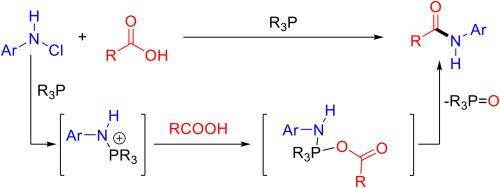
膦介导的羧酸和n -氯化物酰胺的脱氧合成
报道了一种利用膦介导的P(V)平台通过羧酸与n -氯化合物脱氧合成酰胺的策略。该方法能够在温和条件下将各种羧酸(包括伯、仲、生物活性分子)以及氨基酸有效地转化为酰胺。该反应的可扩展性突出了其在大规模酰胺生产中的实际适用性。这种方法为活化试剂提供了一个新的视角,侧重于苯胺活化而不是传统的羧酸活化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


