Multi-level validation of high expression of PLAUR, FOSL2, and SLC11A1 in aortic dissection and their clinical implications
IF 4.7
2区 医学
Q2 IMMUNOLOGY
引用次数: 0
Abstract
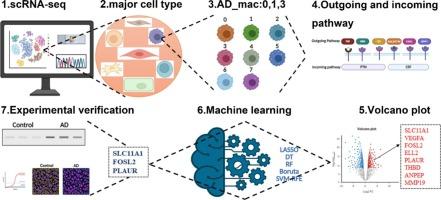
Macrophages, pivotal orchestrators of the immune system, are integral to the initiation of specific immune responses and exert profound influence on the pathogenesis, progression, and therapeutic landscape of aortic dissection (AD). Leveraging the precision of single-cell RNA sequencing (scRNA-seq), this study aimed to dissect the heterogeneity of macrophages within the AD microenvironment. We identified a unique macrophage subpopulation, termed AD-associated macrophages (AD-mac), which is predominantly implicated in the early stages of AD pathogenesis.
To unravel the functional and regulatory underpinnings of AD-mac, we employed a multifaceted analytical approach, integrating advanced computational tools such as CellChat for intercellular communication analysis, Monocle for pseudotemporal trajectory inference, and CytoTRACE for cellular potency assessment. Furthermore, high-dimensional weighted gene co-expression network analysis (hdWGCNA) enabled the identification of a gene module closely associated with this macrophage subpopulation. To distill the most salient molecular signatures of AD, we applied a robust ensemble of machine learning algorithms, including Least Absolute Shrinkage and Selection Operator (LASSO), Support Vector Machine with Recursive Feature Elimination (SVM-RFE), Random Forest (RF), Boruta, and Decision Tree (DT), to bulk RNA-seq data. This integrative approach revealed a panel of characteristic AD genes. Validation studies were conducted using aortic tissue samples from human AD patients and a murine AD model. Notably, we observed significant upregulation of PLAUR, FOSL2, and SLC11A1 at both mRNA and protein levels within the dissected tissues, a finding further substantiated by immunohistochemical staining in the murine model. Immunofluorescence analysis confirmed the colocalization of PLAUR, FOSL2, and SLC11A1 with the macrophage marker CD68, underscoring their expression within the AD-mac subpopulation. In summary, this study delineates the critical pathogenic macrophage subpopulations and their regulatory gene networks in AD, providing a foundational framework for the development of novel diagnostic biomarkers and therapeutic targets. These insights hold significant promise for advancing the clinical management of aortic dissection.
PLAUR、FOSL2和SLC11A1在主动脉夹层中高表达的多层次验证及其临床意义
巨噬细胞是免疫系统的关键协调者,是特异性免疫反应启动不可或缺的一部分,对主动脉夹层(AD)的发病、进展和治疗前景有着深远的影响。利用单细胞RNA测序(scRNA-seq)的精度,本研究旨在剖析AD微环境中巨噬细胞的异质性。我们发现了一种独特的巨噬细胞亚群,称为AD相关巨噬细胞(AD-mac),它主要与AD发病的早期阶段有关。为了揭示AD-mac的功能和调控基础,我们采用了多方面的分析方法,整合了先进的计算工具,如用于细胞间通信分析的CellChat,用于伪时间轨迹推断的Monocle和用于细胞效力评估的CytoTRACE。此外,高维加权基因共表达网络分析(hdWGCNA)能够鉴定出与该巨噬细胞亚群密切相关的基因模块。为了提取AD最显著的分子特征,我们应用了一个强大的机器学习算法集合,包括最小绝对收缩和选择算子(LASSO),支持向量机递归特征消除(SVM-RFE),随机森林(RF), Boruta和决策树(DT),以批量RNA-seq数据。这种综合方法揭示了一组典型的阿尔茨海默病基因。使用人类AD患者和小鼠AD模型的主动脉组织样本进行验证研究。值得注意的是,我们在解剖组织中观察到PLAUR、FOSL2和SLC11A1 mRNA和蛋白水平的显著上调,这一发现在小鼠模型中得到了免疫组化染色的进一步证实。免疫荧光分析证实PLAUR、FOSL2和SLC11A1与巨噬细胞标记物CD68共定位,强调它们在AD-mac亚群中的表达。总之,本研究描述了AD的关键致病巨噬细胞亚群及其调控基因网络,为开发新的诊断生物标志物和治疗靶点提供了基础框架。这些见解对推进主动脉夹层的临床管理具有重要的意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.40
自引率
3.60%
发文量
935
审稿时长
53 days
期刊介绍:
International Immunopharmacology is the primary vehicle for the publication of original research papers pertinent to the overlapping areas of immunology, pharmacology, cytokine biology, immunotherapy, immunopathology and immunotoxicology. Review articles that encompass these subjects are also welcome.
The subject material appropriate for submission includes:
• Clinical studies employing immunotherapy of any type including the use of: bacterial and chemical agents; thymic hormones, interferon, lymphokines, etc., in transplantation and diseases such as cancer, immunodeficiency, chronic infection and allergic, inflammatory or autoimmune disorders.
• Studies on the mechanisms of action of these agents for specific parameters of immune competence as well as the overall clinical state.
• Pre-clinical animal studies and in vitro studies on mechanisms of action with immunopotentiators, immunomodulators, immunoadjuvants and other pharmacological agents active on cells participating in immune or allergic responses.
• Pharmacological compounds, microbial products and toxicological agents that affect the lymphoid system, and their mechanisms of action.
• Agents that activate genes or modify transcription and translation within the immune response.
• Substances activated, generated, or released through immunologic or related pathways that are pharmacologically active.
• Production, function and regulation of cytokines and their receptors.
• Classical pharmacological studies on the effects of chemokines and bioactive factors released during immunological reactions.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


