Green construction of porous organic polymers based on tris(β-keto-hydrazo)-cyclohexane used for the fluorescence detection of iodide ions and 2,4-dinitrophenol
IF 4.2
3区 工程技术
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
The porous organic polymers based on tris(β-keto-hydrazo)-cyclohexane (the TKH-POPs) are new POPs, which are synthesized by the simple diazo-coupling isomerization reactions in aqueous solution at room temperature. The synthesis process is green, mild, and environment-friendly, which avoids the use of metal catalyst, high temperature, and toxic organic reagents. However, few studies have reported their use as fluorescent sensors up to now. Herein, two TKH-POPs (PDADM and PTDA) were synthesized with 4,4′-diaminodiphenylmethane, 4,4′-thiodianiline, and anhydrous phloroglucinol as the building blocks. The structures of PDADM and PTDA are the tris(β-keto-hydrazo)-cyclohexane forms rather than the azo tautomers. PDADM and PTDA are amorphous nanospheres with thermal stability up to 264 and 242 °C, and high BET surface areas of 1346 and 989 m2 g−1. PDADM and PTDA have high sensitivity and selectivity to iodide ion (I−) and 2,4-dinitrophenol (DNP). The KSV values of PDADM and PTDA are 1.47 × 104 and 2.41 × 104 mol L−1 for I−, and 1.89 × 104 and 2.68 × 104 L mol−1 for DNP. The fluorescence sensing mechanisms of PDADM and PTDA to iodide ions include the heavy atom effect, inter filtration effect, and resonance energy transfer process, and that to DNP consist of resonance energy transfer process, photo-induced electron-transfer process, inter filtration effect, and hydrogen bonding interactions. This study lays a good foundation for the use of TKH-POPs as the fluorescent sensors to detect anions and nitro aromatic compounds.
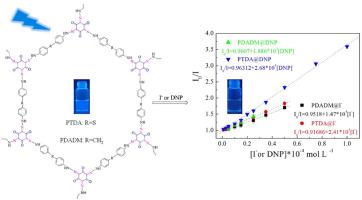
基于三(β-酮腙)环己烷的多孔有机聚合物的绿色构建,用于碘离子和2,4-二硝基苯酚的荧光检测
以三(β-酮-腙)环己烷为基的多孔有机聚合物(TKH-POPs)是一种在室温水溶液中通过简单的重氮偶联异构化反应合成的新型POPs。合成过程绿色、温和、环保,避免了金属催化剂、高温、有毒有机试剂的使用。然而,迄今为止,很少有研究报道将其用作荧光传感器。本文以4,4′-二氨基二苯甲烷、4,4′-硫代二苯胺和无水间苯三酚为原料合成了两种tkh - pop (PDADM和PTDA)。PDADM和PTDA的结构是三(β-酮-腙)-环己烷形式,而不是偶氮互变异构体。PDADM和PTDA均为非晶纳米球,热稳定性可达264℃和242℃,BET表面积高达1346和989 m2 g−1。PDADM和PTDA对碘离子(I−)和2,4-二硝基苯酚(DNP)具有较高的敏感性和选择性。PDADM和PTDA对I−的KSV值分别为1.47 × 104和2.41 × 104 mol L−1,对DNP的KSV值分别为1.89 × 104和2.68 × 104 L mol−1。PDADM和PTDA对碘离子的荧光感应机制包括重原子效应、间滤效应和共振能量转移过程,对DNP的荧光感应机制包括共振能量转移过程、光致电子转移过程、间滤效应和氢键相互作用。本研究为利用TKH-POPs作为荧光传感器检测阴离子和硝基芳香族化合物奠定了良好的基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Dyes and Pigments
工程技术-材料科学:纺织
CiteScore
8.20
自引率
13.30%
发文量
933
审稿时长
33 days
期刊介绍:
Dyes and Pigments covers the scientific and technical aspects of the chemistry and physics of dyes, pigments and their intermediates. Emphasis is placed on the properties of the colouring matters themselves rather than on their applications or the system in which they may be applied.
Thus the journal accepts research and review papers on the synthesis of dyes, pigments and intermediates, their physical or chemical properties, e.g. spectroscopic, surface, solution or solid state characteristics, the physical aspects of their preparation, e.g. precipitation, nucleation and growth, crystal formation, liquid crystalline characteristics, their photochemical, ecological or biological properties and the relationship between colour and chemical constitution. However, papers are considered which deal with the more fundamental aspects of colourant application and of the interactions of colourants with substrates or media.
The journal will interest a wide variety of workers in a range of disciplines whose work involves dyes, pigments and their intermediates, and provides a platform for investigators with common interests but diverse fields of activity such as cosmetics, reprographics, dye and pigment synthesis, medical research, polymers, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


