Impact of firing temperature and atmosphere on the chemical reactivity of UO2+x powders in nitric acid
IF 4.8
2区 材料科学
Q1 METALLURGY & METALLURGICAL ENGINEERING
引用次数: 0
Abstract
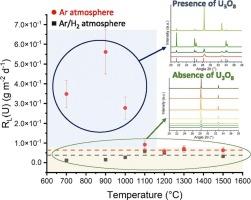
The aim of this work is to study the dissolution kinetics of a series of powdered UO2+x samples with different structural and microstructural properties. For this purpose, UO2+x powders were prepared by hydroxide precipitation and then heat-treated at different temperatures under argon and reducing atmospheres. The calcined UO2+x samples were first investigated ex-situ by several physicochemical techniques in order to highlight the dependence of the normalized dissolution rates on various parameters. The PXRD experiments showed the preservation of the fluorite structure under reducing atmosphere over the whole temperature range studied, while the formation of a U3O8 phase was highlighted under argon at T ≤ 1100 °C. The study of the dissolution of UO2+x samples first highlighted the effect of increasing the calcination temperature (decrease of SSA), which significantly improves the chemical durability of the solids. The higher the calcination temperature, the lower the reactivity of the sample and the longer the time required to reach full dissolution. Secondly, the presence of a U3O8 fraction in some samples calcined under argon resulted in a higher normalized dissolution rate. For comparison, the normalized dissolution rate of a pure U3O8 sample reached RL = (5.6 ± 1.1) × 10−1 g m−2 d−1, a higher value than that of UO2+x, RL = 5.5 × 10−2 g m−2 d−1 on average. Furthermore, these samples showed no change in kinetic regime during dissolution, which could be explained by the blocking by U3O8 of the transition to a kinetic dissolution regime autocatalyzed by nitrogen species.
烧制温度和气氛对UO2+x粉末在硝酸中化学反应性的影响
本研究的目的是研究一系列具有不同结构和微观结构性质的UO2+x粉末样品的溶解动力学。为此,采用氢氧化物沉淀法制备了UO2+x粉末,并在氩气和还原气氛下进行了不同温度的热处理。为了突出标准化溶解速率对各种参数的依赖性,我们首先通过几种物理化学技术对煅烧的UO2+x样品进行了非原位研究。PXRD实验表明,在整个研究温度范围内,还原性气氛下,萤石结构得以保存,而在T≤1100℃的氩气条件下,U3O8相的形成较为突出。UO2+x样品的溶解研究首先突出了提高煅烧温度(降低SSA)的效果,显著提高了固体的化学耐久性。煅烧温度越高,样品的反应性越低,达到完全溶解所需的时间越长。其次,在氩气下煅烧的一些样品中存在U3O8馏分,导致了更高的归一化溶解速率。相比之下,U3O8纯样品的标准化溶解速率RL =(5.6±1.1)× 10−1 g m−2 d−1,高于UO2+x的平均RL = 5.5 × 10−2 g m−2 d−1。此外,这些样品在溶解过程中没有表现出动力学模式的变化,这可以解释为U3O8阻断了向氮自催化的动力学溶解模式的转变。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Hydrometallurgy
工程技术-冶金工程
CiteScore
9.50
自引率
6.40%
发文量
144
审稿时长
3.4 months
期刊介绍:
Hydrometallurgy aims to compile studies on novel processes, process design, chemistry, modelling, control, economics and interfaces between unit operations, and to provide a forum for discussions on case histories and operational difficulties.
Topics covered include: leaching of metal values by chemical reagents or bacterial action at ambient or elevated pressures and temperatures; separation of solids from leach liquors; removal of impurities and recovery of metal values by precipitation, ion exchange, solvent extraction, gaseous reduction, cementation, electro-winning and electro-refining; pre-treatment of ores by roasting or chemical treatments such as halogenation or reduction; recycling of reagents and treatment of effluents.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


