Enantioselective Ni-catalyzed reductive cross-coupling of propargyl halides with vinyl halides to skipped enynes
引用次数: 0
Abstract
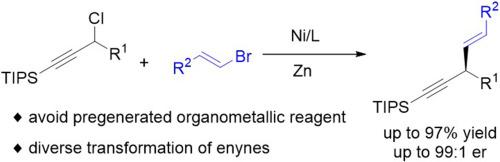
Skipped enynes represent valuable structural motifs in synthetic chemistry due to their unique reactivity and prevalence in complex molecules. Herein, we describe a streamlined approach for the enantioselective synthesis of chiral skipped enynes via nickel-catalyzed asymmetric reductive cross-coupling between propargyl halides and vinyl halides. This transformation offers several notable advantages, including the use of readily available starting materials, mild reaction conditions, broad functional group tolerance, and potential for diverse downstream functionalizations.
镍催化异丙基卤化物与乙烯基卤化物的对映选择性交叉偶联反应
跳过炔由于其独特的反应活性和在复杂分子中的普遍存在,在合成化学中代表了有价值的结构基序。在此,我们描述了一种简化的方法,通过镍催化丙炔卤化物和乙烯基卤化物之间的不对称还原交叉偶联来对映选择性合成手性跳过烯。这种转化有几个显著的优点,包括使用现成的起始材料,温和的反应条件,广泛的官能团耐受性,以及多种下游功能化的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


