An undescribed phytyl melilotic acid ester and chromomoric acid derivatives from Artemisia anomala and their cytotoxic and anti-inflammatory activities
IF 2.6
3区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Phytochemical investigation of the ethanol extract from Artemisia anomala twigs and leaves led to the isolation and structural elucidation of four previously unreported compounds, including a phytyl melilotic acid ester designated as artemanoin A (1), and three chromomoric acid derivatives named anomalones E − G (2–4). Their structures, including the absolute configurations, were unambiguously established through extensive spectroscopic characterization (HRESIMS, 1D/2D NMR) coupled with chemical derivatization study and quantum chemical calculations (including ECD, optical rotation and NMR computations). Notably, artemanoin A (1) represents an acyclic diterpenoid featuring an unusual ester linkage with melilotic acid, a structural motif rarely encountered in natural products. In biological evaluations, while none of the isolated compounds exhibited cytotoxic activity against the tested cancer cell lines at concentrations up to 50 μM, compounds 2–4 demonstrated significant anti-inflammatory potential, showing potent inhibition of NO production in LPS-stimulated RAW264.7 macrophages with IC50 values of 2.81–4.13 μM.
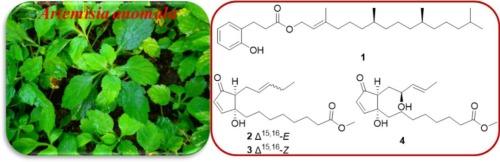
一种未描述的黄花蒿叶基二苯乙烯酸酯和铬酸衍生物及其细胞毒性和抗炎活性
通过对青蒿(Artemisia anomalala)细枝和叶片乙醇提取物的植物化学研究,分离并鉴定了四种以前未报道过的化合物,包括一种被命名为artemanoin a(1)的植基melilotic酸酯,以及三种被命名为anomalones E−G(2-4)的色酸衍生物。它们的结构,包括绝对构型,通过广泛的光谱表征(hresms, 1D/2D NMR),结合化学衍生化研究和量子化学计算(包括ECD,旋光性和核磁共振计算)明确地建立起来。值得注意的是,artemanoin A(1)是一种无环二萜类化合物,与melilotic acid具有不寻常的酯连接,这是一种在天然产物中很少遇到的结构基序。在生物学评价中,虽然分离的化合物在50 μM浓度下对所测试的癌细胞没有细胞毒性活性,但化合物2-4显示出显著的抗炎潜力,显示出对lps刺激的RAW264.7巨噬细胞NO产生的有效抑制,IC50值为2.81-4.13 μM。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Fitoterapia
医学-药学
CiteScore
5.80
自引率
2.90%
发文量
198
审稿时长
1.5 months
期刊介绍:
Fitoterapia is a Journal dedicated to medicinal plants and to bioactive natural products of plant origin. It publishes original contributions in seven major areas:
1. Characterization of active ingredients of medicinal plants
2. Development of standardization method for bioactive plant extracts and natural products
3. Identification of bioactivity in plant extracts
4. Identification of targets and mechanism of activity of plant extracts
5. Production and genomic characterization of medicinal plants biomass
6. Chemistry and biochemistry of bioactive natural products of plant origin
7. Critical reviews of the historical, clinical and legal status of medicinal plants, and accounts on topical issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


