Irf4 participates in benzene-induced hematopoietic senescence through mitochondrial ROS-dependent BCAA catabolism
IF 4.6
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
The continuous accumulation of senescent hematopoietic stem progenitors (HSPCs) contributes to hematopoietic damage. Benzene is a confirmed human carcinogen, and its damage to HSPCs is a key event in benzene poisoning. However, whether the environmental dose of benzene is involved in HSPC damage by inducing cellular senescence has not been reported. Here, male C57BL/6 J mice were exposed to benzene vapour for 12 weeks (0, 10, 50 ppm), and mouse hematopoietic progenitor cell line FDC-P1 was exposed to benzene metabolite 1,4-BQ for 24 h (0, 5, 10 μM). In vivo and in vitro models combined with single-cell RNA sequencing have shown that benzene and its metabolites caused senescence in whole bone marrow and hematopoietic progenitor cells. Proteomics showed that benzene exposure significantly up-regulated interferon regulatory factor 4 (Irf4) in the bone marrow. Irf4 inhibition alleviated cellular senescence and hematopoietic damage, suggesting that Irf4 is a key molecule in benzene-induced hematopoietic cell senescence. Mechanistically, benzene caused a decrease in branched-chain amino acids (BCAAs) in whole bone marrow, hematopoietic progenitor cells, and plasma, and an increase in the BCAA catabolic enzyme Bcat1, mitochondrial ROS, and Bckdh activity. Irf4 inhibition down-regulated Bcat1, alleviated mitochondrial oxidative stress-dependent Bckdh abnormality, and up-regulated BCAAs. BCAA supplementation effectively alleviated benzene-induced cellular senescence and hematopoietic damage. In conclusion, the study identified that Irf4 triggered benzene-induced hematopoietic progenitor cell senescence and hematopoietic damage by mitochondrial injury-induced excessive BCAA catabolism. This study provides new ideas on the molecular mechanism of benzene-induced hematopoietic damage from the perspective of metabolism and senescence.
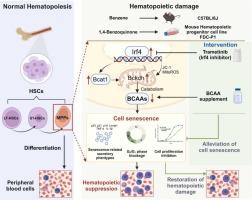
Irf4通过线粒体ros依赖性BCAA分解代谢参与苯诱导的造血衰老
衰老造血干细胞祖细胞(HSPCs)的持续积累导致造血损伤。苯是公认的人类致癌物,其对HSPCs的损害是苯中毒的关键事件。然而,环境剂量苯是否通过诱导细胞衰老参与HSPC损伤尚未见报道。在这里,雄性C57BL/6 J小鼠暴露于苯蒸气(0,10,50 ppm) 12周,小鼠造血祖细胞系FDC-P1暴露于苯代谢物1,4- bq 24 h(0,5,10 μM)。结合单细胞RNA测序的体内和体外模型表明,苯及其代谢物导致整个骨髓和造血祖细胞衰老。蛋白质组学显示,苯暴露显著上调骨髓中的干扰素调节因子4 (Irf4)。抑制Irf4可减轻细胞衰老和造血损伤,提示Irf4是苯诱导的造血细胞衰老的关键分子。机制上,苯引起全骨髓、造血祖细胞和血浆中支链氨基酸(BCAAs)的减少,BCAA分解代谢酶Bcat1、线粒体ROS和Bckdh活性的增加。抑制Irf4可下调Bcat1,缓解线粒体氧化应激依赖性Bckdh异常,上调BCAAs。补充支链氨基酸可有效缓解苯诱导的细胞衰老和造血损伤。综上所述,本研究发现Irf4通过线粒体损伤引起的过量BCAA分解代谢引发苯诱导的造血祖细胞衰老和造血损伤。本研究从代谢和衰老的角度对苯诱导造血损伤的分子机制提供了新的思路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Toxicology
医学-毒理学
CiteScore
7.80
自引率
4.40%
发文量
222
审稿时长
23 days
期刊介绍:
Toxicology is an international, peer-reviewed journal that publishes only the highest quality original scientific research and critical reviews describing hypothesis-based investigations into mechanisms of toxicity associated with exposures to xenobiotic chemicals, particularly as it relates to human health. In this respect "mechanisms" is defined on both the macro (e.g. physiological, biological, kinetic, species, sex, etc.) and molecular (genomic, transcriptomic, metabolic, etc.) scale. Emphasis is placed on findings that identify novel hazards and that can be extrapolated to exposures and mechanisms that are relevant to estimating human risk. Toxicology also publishes brief communications, personal commentaries and opinion articles, as well as concise expert reviews on contemporary topics. All research and review articles published in Toxicology are subject to rigorous peer review. Authors are asked to contact the Editor-in-Chief prior to submitting review articles or commentaries for consideration for publication in Toxicology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


