The utilization of non-human primates in the investigation of autism spectrum disorder
IF 2.6
4区 医学
Q3 NEUROSCIENCES
引用次数: 0
Abstract
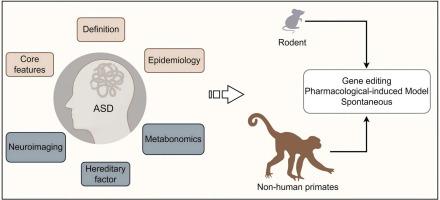
Autism spectrum disorder (ASD), a prevalent neurodevelopmental condition characterized by social communication deficits and repetitive behaviors, presents significant therapeutic challenges due to its multifactorial etiology, clinical heterogeneity, and frequent comorbidities. To address these complexities, animal models have become indispensable tools for unraveling ASD pathogenesis and evaluating potential interventions. This review synthesizes recent advances across three pivotal research domains—neuroimaging biomarkers, metabolic dysregulation, and etiological mechanisms—while providing a critical evaluation of animal models, including rodent and non-human primate (NHP) paradigms developed through pharmacological induction, spontaneous mutations, and CRISPR-based gene editing. Although rodent models have substantially advanced our understanding of ASD-linked genetic pathways and neural circuitry, their limited capacity to model higher-order social cognition underscores the need for evolutionarily proximate systems. Non-human primates (NHPs), with their neuroanatomical homology to humans and complex socio-cognitive behaviors, offer unparalleled advantages for recapitulating core ASD phenotypes. Current evidence demonstrates that NHP models faithfully replicate hallmark behavioral manifestations while elucidating aberrant neural network dynamics and synaptic plasticity underlying these traits. Key challenges in the field include standardizing model validation protocols, addressing sex-specific phenotypic variability, and integrating multi-omics approaches for biomarker discovery and circuit-level analysis, and evaluating the translational utility of NHP models.
非人类灵长类动物在自闭症谱系障碍研究中的应用
自闭症谱系障碍(ASD)是一种以社会沟通缺陷和重复行为为特征的普遍神经发育疾病,由于其多因素病因、临床异质性和常见的合并症,给治疗带来了重大挑战。为了解决这些复杂性,动物模型已成为揭示ASD发病机制和评估潜在干预措施不可或缺的工具。本综述综合了三个关键研究领域的最新进展-神经成像生物标志物,代谢失调和病因机制-同时提供了对动物模型的关键评估,包括通过药物诱导,自发突变和基于crispr的基因编辑开发的啮齿动物和非人灵长类动物(NHP)范式。尽管啮齿类动物模型极大地促进了我们对自闭症相关遗传途径和神经回路的理解,但它们在模拟高阶社会认知方面的能力有限,这突显了对进化近似系统的需求。非人灵长类动物(NHPs)具有与人类相似的神经解剖学和复杂的社会认知行为,为概括核心ASD表型提供了无与伦比的优势。目前的证据表明,NHP模型忠实地复制了标志性的行为表现,同时阐明了这些特征背后的异常神经网络动力学和突触可塑性。该领域的主要挑战包括标准化模型验证协议,解决性别特异性表型变异性,整合多组学方法进行生物标志物发现和电路水平分析,以及评估NHP模型的转化效用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Brain Research
医学-神经科学
CiteScore
5.90
自引率
3.40%
发文量
268
审稿时长
47 days
期刊介绍:
An international multidisciplinary journal devoted to fundamental research in the brain sciences.
Brain Research publishes papers reporting interdisciplinary investigations of nervous system structure and function that are of general interest to the international community of neuroscientists. As is evident from the journals name, its scope is broad, ranging from cellular and molecular studies through systems neuroscience, cognition and disease. Invited reviews are also published; suggestions for and inquiries about potential reviews are welcomed.
With the appearance of the final issue of the 2011 subscription, Vol. 67/1-2 (24 June 2011), Brain Research Reviews has ceased publication as a distinct journal separate from Brain Research. Review articles accepted for Brain Research are now published in that journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


