Functional connectivity linking the hippocampus, retrosplenial, and orbitofrontal cortex correlates with increased seizure severity in temporal lobe epilepsy
IF 2.6
4区 医学
Q3 NEUROSCIENCES
引用次数: 0
Abstract
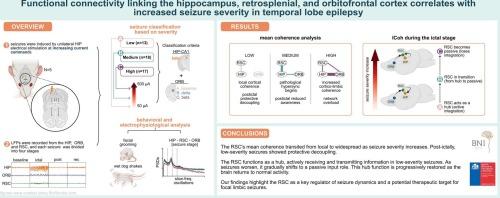
Temporal lobe epilepsy (TLE) is a highly prevalent neurological disorder characterized by severe seizures and altered consciousness. Seizures in TLE often originate in the hippocampus (HIP) but can spread to widespread brain regions, including the retrosplenial cortex (RSC) and orbitofrontal cortex (ORB). The RSC is a highly interconnected cortical region implicated in seizure onset and propagation, yet its role in epilepsy remains poorly understood. We induced seizures (n = 48) in behaving adult male Sprague-Dawley rats (n = 5) via unilateral electrical stimulation of the right ventral hippocampus (targeting the dentate gyrus) using square biphasic pulses (1 ms per phase) at 60 Hz for 2 s. Stimulation began at 50 μA and was increased in 25 μA steps if no seizure was evoked, up to a maximum of 500 μA or until the animals exhibited up to five seizure episodes, to investigate the dynamic changes in the RSC during seizures of varying severity and their temporal coordination with other brain regions.
Seizures were categorized based on the presence of fast poly-spike activity (7–15 Hz) and slow-wave activity (1–2 Hz) and their spread to the HIP and ORB. Spectral power density changes across the three regions correlated with seizure severity. Coherence patterns transitioned from adaptive (flexible and localized), RSC-ORB and HIP-RSC coupling to maladaptive, persistent hypersynchronization involving the HIP-RSC and HIP-ORB hypersynchronization as severity increased.
The RSC acts as a key hub for integrating and transmitting information between distant brain regions, particularly during low-severity seizures. In contrast, it predominantly receives input from both upstream and downstream sources during high-severity seizures. Our findings provide critical insights into the relationship between seizure severity and brain network dynamics, highlighting the RSC as a potential therapeutic target for epilepsy.
连接海马、脾后和眶额皮质的功能连通性与颞叶癫痫发作严重程度增加相关
颞叶癫痫(TLE)是一种非常普遍的神经系统疾病,其特征是严重的癫痫发作和意识改变。TLE的癫痫发作通常起源于海马体(HIP),但可以扩散到广泛的大脑区域,包括脾后皮质(RSC)和眶额皮质(ORB)。RSC是一个高度互联的皮质区域,与癫痫发作和传播有关,但其在癫痫中的作用仍知之甚少。我们使用方形双相脉冲(每相1 ms) 60 Hz持续2 s电刺激右侧腹侧海马(针对齿状回),诱导成年雄性spraguedawley大鼠(n = 5)癫痫发作(n = 48)。从50 μA开始刺激,如果没有癫痫发作,则以25 μA的步骤增加刺激,最大刺激到500 μA或直到动物出现多达5次癫痫发作,以研究不同程度癫痫发作期间RSC的动态变化及其与其他脑区域的时间协调。根据快速多峰活动(7 - 15hz)和慢波活动(1 - 2hz)的存在及其向HIP和ORB的扩散来分类癫痫发作。三个区域的频谱功率密度变化与癫痫发作的严重程度相关。相干模式随着严重程度的增加,从自适应(灵活和局部)、RSC-ORB和HIP-RSC耦合转变为包括HIP-RSC和HIP-ORB超同步的不适应、持续超同步。RSC是在远端大脑区域之间整合和传递信息的关键枢纽,尤其是在轻度癫痫发作期间。相反,在高度严重的癫痫发作期间,它主要接收来自上游和下游来源的输入。我们的研究结果为癫痫发作严重程度和脑网络动态之间的关系提供了重要的见解,突出了RSC作为癫痫的潜在治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Brain Research
医学-神经科学
CiteScore
5.90
自引率
3.40%
发文量
268
审稿时长
47 days
期刊介绍:
An international multidisciplinary journal devoted to fundamental research in the brain sciences.
Brain Research publishes papers reporting interdisciplinary investigations of nervous system structure and function that are of general interest to the international community of neuroscientists. As is evident from the journals name, its scope is broad, ranging from cellular and molecular studies through systems neuroscience, cognition and disease. Invited reviews are also published; suggestions for and inquiries about potential reviews are welcomed.
With the appearance of the final issue of the 2011 subscription, Vol. 67/1-2 (24 June 2011), Brain Research Reviews has ceased publication as a distinct journal separate from Brain Research. Review articles accepted for Brain Research are now published in that journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


