Amide hemiaminals from retro-Claisen ring opening of chiral 2-siloxy-1-cyclobutene-1-carboxamides
IF 2.2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Nucleophilic retro-Claisen ring opening of chiral cis-3,4-disubstituted cyclobutene-1-carboxamides with amines forms cyclic hemiaminals in good yield with complete retention of configuration. The chiral cyclobutene-1-carboxamides are prepared by [3 + 1]-cycloaddition of donor-acceptor 2-silyloxy-1-cyclopropene-1-carboxamides by copper(I) catalysis with chiral SaBox ligands with high cis selectivity in modest yields and enantioselectivities. Retro-Claisen ring opening by benzylamine formed the cyclic hemiaminal as the dominant product, but ring opening by cyclohexylamine gave the open form as the dominant product. The cyclic hemiaminal and open form are not interconvertible under the reaction conditions.
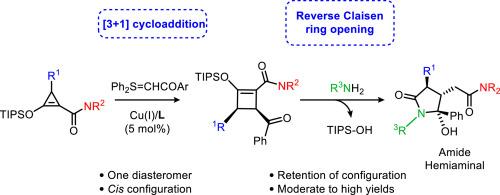
手性2-硅氧基-1-环丁烯-1-羧酰胺的反claisen开环酰胺半聚物
手性顺式-3,4-二取代环丁烯-1-羧酸酰胺与胺的亲核反克拉森开环反应产率高,构型完全保留。以铜(I)为催化剂,用手性SaBox配体对2-硅氧基-1-环丙烯-1-羧酰胺进行[3 + 1]环加成反应,制备了手性环丁烯-1-羧酰胺,产率中等,对映选择性高。苯胺开环的优势产物为环半胺,而环己胺开环的优势产物为开环。在反应条件下,环半胺型和开型不能相互转化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


