Duración de la deprivación androgénica con la radioterapia de rescate en los pacientes con cáncer de próstata con recidiva bioquímica tras cirugía: datos iniciales de reclutamiento en el ensayo fase III URONCOR 06-24
IF 1.2
4区 医学
Q3 UROLOGY & NEPHROLOGY
引用次数: 0
Abstract
Introduction
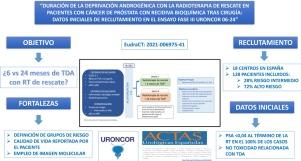
URONCOR 06-24 (NCT05781217) is a prospective, multicenter, randomized, open-label, phase III trial evaluating the impact on distant metastasis-free survival (MFS) of short-term (6 months) versus long-term (24 months) androgen deprivation therapy (ADT) in combination with salvage radiotherapy in high- and intermediate-risk patients after biochemical recurrence (BCR).
Material and method
A total of 534 men will be randomized to receive either 6 or 24 months of ADT. Stratification is based on risk group (intermediate vs high) and nodal status (pN0 vs pNx).
Results
From March 2023 to November 2024, 122 patients have been enrolled: 34 (28%) with intermediate risk and 88 (72%) with high risk. Fifty-five patients (45%) are pNx. The mean time from surgery to BCR is 25.4 months, and the PSA at inclusion was 0.55 ng/ml. Restaging was performed in 89 patients, 75 of whom underwent PET/CT (97%, PSMA PET/CT). Hypofractionation was used in 68% of cases, and elective pelvic irradiation in 33%. At the time of analysis, all patients had PSA normalization. No severe ADT-related toxicity has been reported
Conclusion
URONCOR 06-24 is the first clinical trial comparing long- versus short-term ADT in the setting of BCR after prostatectomy, with stratification by risk group. Initial recruitment data show a balanced distribution of prognostic factors between both arms and no serious adverse events related to ADT.
手术后生化复发的前列腺癌患者在进行挽救性放射治疗的同时剥夺雄激素的时间长短:III期试验初步招募数据URONCOR 06-24
uroncor 06-24 (NCT05781217)是一项前瞻性、多中心、随机、开放标签的III期临床试验,评估短期(6个月)与长期(24个月)雄激素剥夺治疗(ADT)联合补补性放疗对生化复发(BCR)后中高危患者远处无转移生存(MFS)的影响。材料和方法总共534名男性将随机接受6个月或24个月的ADT治疗。分层是基于风险组(中等vs高)和淋巴结状态(pN0 vs pNx)。结果从2023年3月至2024年11月共纳入122例患者,其中中危34例(28%),高危88例(72%)。55例(45%)为pNx。从手术到BCR平均时间为25.4个月,纳入时PSA为0.55 ng/ml。89例患者进行了重新分期,其中75例接受了PET/CT(97%为PSMA PET/CT)。68%的病例采用低分割术,33%的病例采用选择性骨盆照射。分析时,所有患者PSA均恢复正常。结论uroncor 06-24是第一个比较前列腺切除术后BCR患者长期和短期ADT的临床试验,并按风险组分层。最初的招募数据显示两组预后因素分布平衡,没有与ADT相关的严重不良事件。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Actas urologicas espanolas
UROLOGY & NEPHROLOGY-
CiteScore
1.90
自引率
0.00%
发文量
98
审稿时长
46 days
期刊介绍:
Actas Urológicas Españolas is an international journal dedicated to urological diseases and renal transplant. It has been the official publication of the Spanish Urology Association since 1974 and of the American Urology Confederation since 2008. Its articles cover all aspects related to urology.
Actas Urológicas Españolas, governed by the peer review system (double blinded), is published online in Spanish and English. Consequently, manuscripts may be sent in Spanish or English and bidirectional free cost translation will be provided.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


