Multi-system toxicity of lead (Pb) in Procambarus clarkii: Integrated analysis of tissue damage, immune dysfunction, oxidative stress, and microbial dysbiosis via multi-omics approaches
IF 4.3
3区 环境科学与生态学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Comparative Biochemistry and Physiology C-toxicology & Pharmacology
Pub Date : 2025-08-27
DOI:10.1016/j.cbpc.2025.110331
引用次数: 0
Abstract
The element lead (Pb) exhibits significant toxicity throughout aquatic habitats. Crustaceans sit at the top of the aquatic food chain and therefore are especially susceptible to Pb-associated toxicity. Nevertheless, there is limited information on the crustaceans exposed to Pb. Hence, in this study, the acute Pb-mediated alterations of histopathology, hepatopancreas transcriptome, and gut microbiomes of Procambarus clarkii (P. clarkii) were performed. Pb exposure caused hepatopancreas damage (lumen dilatation, epithelial vacuolization) and intestinal lesions (disrupted lamina propria and epithelium). RNA-seq revealed differentially expressed genes (DEGs) in the hepatopancreas, including immune-related (PGRMC1, TAK1, CDC42, USP2), oxidation-reduction-related (XDH, G6PD, CYP4C1, CYP6K1, CYP9E2), and ion transport genes (TNC2, LETM1, ABCA1/3/10), suggesting Pb disrupts immunity, oxidation-reduction balance, and ion homeostasis. Additionally, examination of the bacterial 16S rRNA gene demonstrated that Pb induced a significantly reduction in both the diversity and abundance of bacteria, resulting in a notable perturbation in the composition of the gut microbiome. The exposure to Pb resulted in significant alterations in 4 phyla and 13 genera. The findings indicated that the presence of Pb might impede the capacity to synthesis of amylases, glucose assimilation, chitin degradation, and antibacterial activity. Taken together, the results of our study provided insights into the multiple mechanisms underlying Pb toxicity in aquatic crustaceans, encompassing tissue, cellular, molecular, and microbial levels.
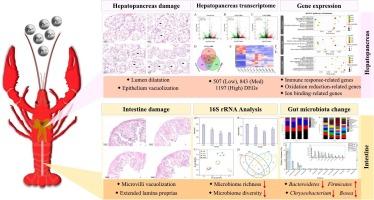
铅在克氏原螯虾中的多系统毒性:通过多组学方法综合分析组织损伤、免疫功能障碍、氧化应激和微生物生态失调
元素铅(Pb)表现出显著的毒性在整个水生生境。甲壳类动物位于水生食物链的顶端,因此特别容易受到铅相关毒性的影响。然而,关于暴露于铅的甲壳类动物的信息有限。因此,本研究对克拉氏原螯虾(P. clarkii)的组织病理学、肝胰脏转录组和肠道微生物组进行了急性铅介导的改变。铅暴露引起肝胰腺损伤(管腔扩张,上皮空泡化)和肠道病变(固有层和上皮破坏)。RNA-seq揭示了肝胰腺中差异表达的基因(DEGs),包括免疫相关基因(PGRMC1、TAK1、CDC42、USP2)、氧化还原相关基因(XDH、G6PD、CYP4C1、CYP6K1、CYP9E2)和离子转运基因(TNC2、LETM1、ABCA1/3/10),表明铅破坏了免疫、氧化还原平衡和离子稳态。此外,对细菌16S rRNA基因的检测表明,铅诱导细菌的多样性和丰度显著降低,导致肠道微生物群组成的显著扰动。Pb暴露对4门13属有显著影响。结果表明,铅的存在可能会阻碍淀粉酶的合成、葡萄糖同化、几丁质降解和抗菌活性。综上所述,我们的研究结果揭示了水生甲壳类动物铅中毒的多种机制,包括组织、细胞、分子和微生物水平。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.50
自引率
5.10%
发文量
206
审稿时长
30 days
期刊介绍:
Part C: Toxicology and Pharmacology. This journal is concerned with chemical and drug action at different levels of organization, biotransformation of xenobiotics, mechanisms of toxicity, including reactive oxygen species and carcinogenesis, endocrine disruptors, natural products chemistry, and signal transduction with a molecular approach to these fields.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


