Characterization and purification of lipovitellin-like egg yolk protein in a stony coral, Acropora aff. tenuis
IF 1.8
3区 生物学
Q4 BIOCHEMISTRY & MOLECULAR BIOLOGY
Comparative Biochemistry and Physiology B-Biochemistry & Molecular Biology
Pub Date : 2025-08-30
DOI:10.1016/j.cbpb.2025.111152
引用次数: 0
Abstract
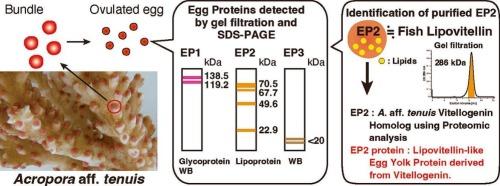
Three egg proteins (EP1, EP2, and EP3) were detected in ovulated eggs of Acropora aff. tenuis, a reef-building stony coral found in tropical and subtropical areas. The proteins were separated into different fractions by gel filtration chromatography, and different patterns were observed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting with antiserum against A. aff. tenuis egg extract (anti-AtE). EP2 was purified from ovulated egg extracts using a combination of hydroxylapatite and gel filtration chromatography. The molecular mass of native EP2 was estimated to be 286 kDa. SDS-PAGE revealed bands at 70.5, 67.7, 49.6, and 22.9 kDa, with the 70.5 and 67.7 kDa bands staining positive for lipid (Sudan black B). These results indicate that purified EP2 is a lipoprotein in A. aff. tenuis eggs, resembling lipovitellin, the major yolk protein derived from the egg yolk precursor, vitellogenin (Vtg), in oviparous vertebrates. Furthermore, EP2 and its bands were digested with trypsin and analyzed by liquid chromatography using a quadrupole time-of-flight tandem mass spectrometer (LC-QTOF-MS). All fragments were identified as A. aff. tenuis Vtg. This is the first report of the purification and characterization of a lipovitellin-like egg yolk protein in non-bilaterian oviparous animals.
石珊瑚(Acropora af . tenuis)卵黄脂样蛋白的鉴定与纯化
在热带和亚热带造礁石珊瑚(Acropora afft . tenuis)的卵细胞中检测到3种卵细胞蛋白(EP1、EP2和EP3)。采用凝胶过滤层析法将蛋白分离成不同的组分,并采用十二烷基硫酸钠-聚丙烯酰胺凝胶电泳(SDS-PAGE)和抗黄颡鱼蛋提取物血清(anti-AtE)进行免疫印迹(Western blotting)检测。用羟基磷灰石和凝胶过滤层析相结合的方法从卵泡提取物中纯化EP2。原生EP2的分子质量估计为286 kDa。SDS-PAGE显示70.5,67.7,49.6和22.9 kDa的条带,其中70.5和67.7 kDa的条带染色为脂质阳性(苏丹黑B)。这些结果表明,纯化后的EP2是黄颡鱼卵中的一种脂蛋白,类似于卵黄蛋白(lipovitellin),后者是卵黄前体卵黄原蛋白(vitellogenin, Vtg)在卵生脊椎动物中的主要卵黄蛋白。用胰蛋白酶消化EP2及其谱带,用四极杆飞行时间串联质谱(LC-QTOF-MS)进行液相色谱分析。所有片段鉴定为A. aff. tenuis Vtg。本文首次报道了非双侧卵生动物卵黄蛋白的纯化和特性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
4.60
自引率
4.50%
发文量
77
审稿时长
22 days
期刊介绍:
Comparative Biochemistry & Physiology (CBP) publishes papers in comparative, environmental and evolutionary physiology.
Part B: Biochemical and Molecular Biology (CBPB), focuses on biochemical physiology, primarily bioenergetics/energy metabolism, cell biology, cellular stress responses, enzymology, intermediary metabolism, macromolecular structure and function, gene regulation, evolutionary genetics. Most studies focus on biochemical or molecular analyses that have clear ramifications for physiological processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


