Engineering zirconium organic gels for fast Li+ conduction
IF 3.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
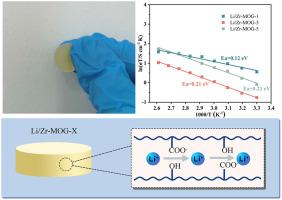
Metal-organic gels (MOGs) are emerging as promising ion‐conductive materials due to their tunable porosity, structural flexibility, and high defect density. In this study, the ionic conduction properties of Zr-MOG and its LiCl-doped derivatives under anhydrous conditions have been investigated. Structural characterization via N2 physisorption reveals that Li + doping reduces long‐range crystallinity and increases specific surface area, thereby facilitating the formation of interconnected ion-transport pathways. In situ infrared spectroscopy confirms the pivotal role of framework carboxylates (R–COO-) and hydroxyls (R–OH) groups in coordinating Li+ and enabling solid‐state ion hopping. Electrochemical impedance spectroscopy demonstrates a significant enhancement in ionic conductivity, with Li/Zr-MOG-3 achieving 1.54 × 10−2 S cm−1 at 383 K under anhydrous conditions, 8-fold higher than pristine Zr-MOG. The low activation energy (0.12–0.21 eV), determined via Arrhenius analysis, indicate an efficient ion hopping mechanism in the absence of water molecules. These findings highlight the potential of defect‐engineered, humidity‐independent Zr-MOG as high‐performance solid‐state Li+ electrolytes, and provide fundamental insight into the role of defect chemistry and functional‐group interactions in optimizing ion mobility.
用于Li+快速传导的工程锆有机凝胶
金属有机凝胶(mog)由于其可调节的孔隙率、结构柔韧性和高缺陷密度而成为一种有前途的离子导电材料。本文研究了Zr-MOG及其掺杂licl衍生物在无水条件下的离子导电性能。通过N2物理吸附进行的结构表征表明,Li +掺杂降低了长程结晶度,增加了比表面积,从而促进了相互连接的离子传输途径的形成。原位红外光谱证实了框架羧酸(R-COO -)和羟基(R-OH)在配位Li+和实现固态离子跳变中的关键作用。电化学阻抗谱表明,Li/Zr-MOG-3的离子电导率显著提高,在383 K无水条件下,Li/Zr-MOG-3的电导率达到1.54 × 10−2 S cm−1,比原始Zr-MOG高8倍。通过阿伦尼乌斯分析确定的低活化能(0.12-0.21 eV)表明,在没有水分子的情况下,离子跳跃机制有效。这些发现突出了缺陷工程、湿度无关的Zr-MOG作为高性能固态Li+电解质的潜力,并为缺陷化学和官能团相互作用在优化迁移率中的作用提供了基本的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Solid State Chemistry
化学-无机化学与核化学
CiteScore
6.00
自引率
9.10%
发文量
848
审稿时长
25 days
期刊介绍:
Covering major developments in the field of solid state chemistry and related areas such as ceramics and amorphous materials, the Journal of Solid State Chemistry features studies of chemical, structural, thermodynamic, electronic, magnetic, and optical properties and processes in solids.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


