Hydrogen bond engineering in aqueous electrolytes: Strategies for cryogenic battery applications
IF 20.2
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Zinc-based and lithium-based battery systems have been extensively utilized in modern electronic devices and automotive applications owing to their exceptional energy density-to-volume ratios. Nevertheless, these energy storage systems employing aqueous electrolytes face critical operational limitations under subzero conditions, where electrolyte crystallization induces severe ionic conductivity degradation and eventual functional failure. Current research efforts exhibit a notable gap in comprehensive mechanistic analyses addressing cryogenic performance deterioration from the perspective of hydrogen bond network disruption. This work presents a systematic review of the fundamental correlation between hydrogen bonding dynamics and phase transition behavior in aqueous media, and the consequent impacts on ion transport mechanisms. The established structure-property relationships offer valuable insights for developing advanced low-temperature aqueous electrolytes through targeted molecular engineering of hydrogen-bond interactions.

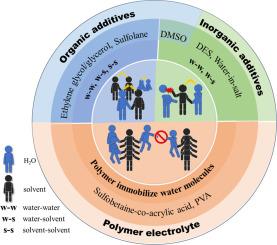
水溶液氢键工程:低温电池应用策略
锌基和锂基电池系统由于其卓越的能量密度与体积比,已广泛应用于现代电子设备和汽车应用中。然而,这些采用水性电解质的储能系统在零下条件下面临着关键的操作限制,电解质结晶会导致严重的离子电导率下降和最终的功能失效。目前的研究在从氢键网络破坏的角度分析低温性能恶化的综合机制方面存在明显的差距。本文对水介质中氢键动力学和相变行为之间的基本关系及其对离子传输机制的影响进行了系统的综述。所建立的结构-性能关系为通过氢键相互作用的靶向分子工程开发先进的低温水溶液电解质提供了有价值的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Energy Storage Materials
Materials Science-General Materials Science
CiteScore
33.00
自引率
5.90%
发文量
652
审稿时长
27 days
期刊介绍:
Energy Storage Materials is a global interdisciplinary journal dedicated to sharing scientific and technological advancements in materials and devices for advanced energy storage and related energy conversion, such as in metal-O2 batteries. The journal features comprehensive research articles, including full papers and short communications, as well as authoritative feature articles and reviews by leading experts in the field.
Energy Storage Materials covers a wide range of topics, including the synthesis, fabrication, structure, properties, performance, and technological applications of energy storage materials. Additionally, the journal explores strategies, policies, and developments in the field of energy storage materials and devices for sustainable energy.
Published papers are selected based on their scientific and technological significance, their ability to provide valuable new knowledge, and their relevance to the international research community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


