High radiosensitivity in the conifer Norway spruce (Picea abies) due to less comprehensive mobilisation of protection and repair responses compared to the radiotolerant Arabidopsis thaliana
IF 6.8
Q1 PLANT SCIENCES
引用次数: 0
Abstract
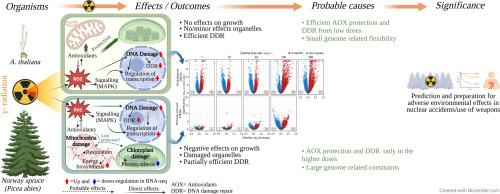
Risk assessment and protection of plant communities in contaminated ecosystems require in-depth understanding of differential sensitivity to chronic ionising radiation in plants. However, the contributing molecular factors to differential radiosensitivity among plant species are poorly understood. To shed light on this, we compared early events associated with protection, repair, and stress responses in gamma-irradiated (1–290 mGy h-1) seedlings of the radiosensitive conifer Norway spruce (Picea abies) and the radiotolerant Arabidopsis thaliana, by analysing growth, organelle and DNA damage, transcriptomes and the dynamics of antioxidant activities and expression of relevant genes. After 48 h of gamma radiation exposure, Norway spruce showed significantly reduced growth at 100–290 mGy h-1 and organelle damage, especially in mitochondria, at ≥ 1 mGy h-1 whereas A. thaliana showed normal vegetative growth at all dose rates, transiently delayed reproductive development at 290 mGy h-1 only, minor organelle damage only at ≥ 100 mGy h-1 and significantly less DNA damage than in Norway spruce at all dose rates. Comparative transcriptomics revealed that A. thaliana showed massive activation of genes related to DNA damage repair, antioxidants, and other stress responses at ≥ 1 mGy h-1 while Norway spruce mobilized transcription of such pathways only at ≥ 40 mGy h-1. The transcriptional activation of repair and protection responses at higher gamma dose-rates only and its absence in lower dose-rates, correlates with high radiosensitivity of Norway spruce, compared to the massive transcriptional activation from low dose-rates in the radiotolerant A. thaliana.
与耐辐射的拟南芥相比,针叶挪威云杉(Picea abies)的高辐射敏感性是由于保护和修复反应的综合动员较少
受污染生态系统中植物群落的风险评估和保护需要深入了解植物对慢性电离辐射的不同敏感性。然而,导致不同植物物种间辐射敏感性差异的分子因素了解甚少。为了阐明这一点,我们通过分析生长、细胞器和DNA损伤、转录组以及抗氧化活性和相关基因表达的动态,比较了辐射敏感针叶树挪威云杉(Picea abies)和耐辐射拟南芥(Arabidopsis thaliana)幼苗在γ辐照(1-290 mGy h-1)下的保护、修复和应激反应的早期事件。照射48 h后,挪威云杉在100 - 290 mGy h-1剂量下的生长显著降低,细胞器损伤(尤其是线粒体)在≥1 mGy h-1剂量下均表现为正常的营养生长,仅在290 mGy h-1剂量下短暂延迟生殖发育,仅在≥100 mGy h-1剂量下细胞器损伤轻微,DNA损伤明显小于挪威云杉。比较转录组学发现,在≥1 mGy h-1时,拟南芥显示出与DNA损伤修复、抗氧化剂和其他应激反应相关的基因的大量激活,而挪威云杉仅在≥40 mGy h-1时才激活这些途径的转录。与耐辐射拟南芥在低剂量下的大量转录激活相比,仅在高剂量率下修复和保护反应的转录激活以及在低剂量率下的缺失与挪威云杉的高辐射敏感性相关。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Plant Stress
PLANT SCIENCES-
CiteScore
5.20
自引率
8.00%
发文量
76
审稿时长
63 days
期刊介绍:
The journal Plant Stress deals with plant (or other photoautotrophs, such as algae, cyanobacteria and lichens) responses to abiotic and biotic stress factors that can result in limited growth and productivity. Such responses can be analyzed and described at a physiological, biochemical and molecular level. Experimental approaches/technologies aiming to improve growth and productivity with a potential for downstream validation under stress conditions will also be considered. Both fundamental and applied research manuscripts are welcome, provided that clear mechanistic hypotheses are made and descriptive approaches are avoided. In addition, high-quality review articles will also be considered, provided they follow a critical approach and stimulate thought for future research avenues.
Plant Stress welcomes high-quality manuscripts related (but not limited) to interactions between plants and:
Lack of water (drought) and excess (flooding),
Salinity stress,
Elevated temperature and/or low temperature (chilling and freezing),
Hypoxia and/or anoxia,
Mineral nutrient excess and/or deficiency,
Heavy metals and/or metalloids,
Plant priming (chemical, biological, physiological, nanomaterial, biostimulant) approaches for improved stress protection,
Viral, phytoplasma, bacterial and fungal plant-pathogen interactions.
The journal welcomes basic and applied research articles, as well as review articles and short communications. All submitted manuscripts will be subject to a thorough peer-reviewing process.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


