Amphiphilic bile acid-amino acid conjugates as sustainable organocatalysts for aqueous asymmetric aldol reactions: From molecular design to aggregation-controlled selectivity
引用次数: 0
Abstract
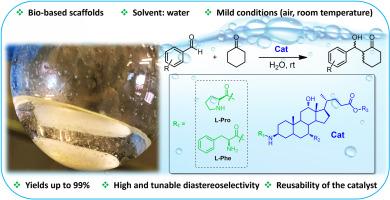
The amine-catalyzed aldol reaction has become a stable of organic synthesis with numerous applications affording predominantly the anti aldol product. Using our synthesized and characterized bile acid-amino acid conjugates with l-proline and l-phenylalanine, and bile acids amino derivatives we successfully performed regioselective aldol reactions in aqueous media controllably giving either the anti or syn diastereomer. These novel derivatives exhibited high catalytic activity, with the l-proline-based organocatalyst showing the highest efficiency, achieving yields up to 99 %, high diastereoselectivity for the anti product and moderate enantioselectivity. The l-phenylalanine-based one demonstrated good catalytic performance, achieving yields up to 99 % and good diastereoselectivity for the syn product. The environmental sustainability of these catalytic systems was further enhanced in the use of bio-based surfactants-amino acids conjugates utilizing water as the reaction medium, without the addition of any cosolvent, while aligning with the modern ethics of environmentally friendly practices. These findings reveal the potential application of natural bile acids scaffolds as versatile and eco-friendly conjugates to afford highly efficiency in sustainable organocatalysis for successfully achieving organic transformations in aqueous environments.
两亲性胆汁酸-氨基酸偶联物作为水不对称醛醇反应的可持续有机催化剂:从分子设计到聚集控制选择性
胺催化的醛醇反应已成为一种稳定的有机合成方法,具有广泛的应用,主要提供抗醛醇产物。利用我们合成和表征的胆汁酸-氨基酸与l-脯氨酸和l-苯丙氨酸的偶联物,以及胆汁酸氨基酸衍生物,我们成功地在水介质中进行了区域选择性醛醇反应,可控制地产生抗或顺非对映体。这些新型衍生物具有较高的催化活性,其中以l-脯氨酸为基础的有机催化剂表现出最高的效率,产率高达99%,抗产物具有较高的非对映选择性和中等的对映选择性。以l-苯丙氨酸为基础的催化产物表现出良好的催化性能,产率高达99%,对合成产物具有良好的非对映选择性。这些催化系统的环境可持续性在使用生物基表面活性剂-氨基酸缀合物时得到进一步增强,利用水作为反应介质,不添加任何助溶剂,同时符合现代环境友好实践的伦理。这些发现揭示了天然胆汁酸支架作为多功能和生态友好的缀合物的潜在应用,为在水环境中成功实现有机转化提供了高效的可持续有机催化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


