Synthesis and structure revision of wasulfisides: glucosyl disulfides isolated from wasabi
IF 2.2
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The first syntheses of three natural glucosyl disulfides—wasulfisides A, B, and 3-epi-wasulfiside B—were successfully accomplished. These compounds were originally isolated from wasabi (Eutrema japonicum) and possess a rare natural β-glucosyl disulfide motif. The core structure was efficiently constructed via S–S bond formation between a β-glucosyl thiotosylate and various thiols. The overall yields reached 37%, starting from d-glucose. Accordingly, the structure of wasulfiside A, a unique oxadithiocine-containing compound, was successfully revised based on the syntheses of its regio- and stereoisomers.
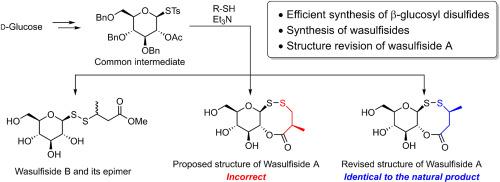
山葵硫化物的合成及结构修正:从山葵中分离的二硫化物葡萄糖基
首次成功地合成了三种天然葡萄糖基二硫化物- wasulfisides A、B和3-epi- wasulfisides B。这些化合物最初是从山葵(Eutrema japonicum)中分离出来的,具有罕见的天然β-葡萄糖基二硫化基序。核心结构通过β-糖基硫代糖酸酯与多种硫醇之间形成S-S键有效构建。从d-葡萄糖开始,总产率达到37%。因此,基于其区域异构体和立体异构体的合成,成功地修改了含有氧二硫辛酸的独特化合物wasulfiside A的结构。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


