Pioneering sulfophosphite crystals: Co-assembly of phosphite and sulfate units in novel inorganic architectures
IF 3.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
Compounds [Ln(HPO3)[S0.5(OH)4] (Ln = La, Ce) and La[HPO2(OH)]SO4·H2O represent the first reported examples of sulfophosphite materials constructed through the co-assembly of HPO3 and SO4 building units. The structures of Ln(HPO3)[S0.5(OH)4] (Ln = La, Ce) feature 2D anionic Ln–P–O layers, composed of 1D Ln–O chains bridged by HPO3 units, which are further linked by S(OH)4 tetrahedra to form its 3D framework. La[HPO2(OH)]SO4·H2O consists of 1D La–S–O chains formed by La2O14 dimers and SO4 tetrahedra, which are further extended into a three-dimensional network via HPO2(OH) linkers. The partial occupancy of sulfur sites in Ln(HPO3)[S0.5(OH)4] (Ln = La, Ce) was validated by energy-dispersive X-ray spectroscopy and X-ray photoelectron spectroscopy. Optical properties investigated through diffuse reflectance spectroscopy, combined with Urbach tail fitting and Tauc plot analysis, revealed that all three compounds exhibit direct band gaps of 4.53, 4.55, and 4.49 eV, respectively. This work not only broadens the structural and compositional diversity of phosphite-based materials but also provides a novel design strategy for the development of functional sulfophosphites with tunable properties.
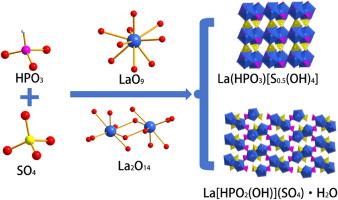
开创性的亚磷酸酯晶体:亚磷酸酯和硫酸盐单位在新型无机结构中的共组装
化合物[Ln(HPO3)[S0.5(OH)4] (Ln = La, Ce)和La[HPO2(OH)]SO4·H2O是首次报道的通过HPO3和SO4构建单元共同组装而成的亚磷酸酯材料。Ln(HPO3)[S0.5(OH)4] (Ln = La, Ce)的结构具有二维阴离子Ln - p - o层,由HPO3单元桥接的1D Ln - o链组成,并由S(OH)4四面体进一步连接形成其三维框架。La[HPO2(OH)]SO4·H2O由La2O14二聚体和SO4四面体形成的一维La - s - o链组成,并通过HPO2(OH)连接体进一步扩展成三维网络。用能量色散x射线能谱和x射线光电子能谱证实了Ln(HPO3)[S0.5(OH)4] (Ln = La, Ce)中硫的部分占据。通过漫反射光谱,结合Urbach尾拟合和Tauc图分析,研究了这三种化合物的光学性质,结果表明,这三种化合物的直接带隙分别为4.53、4.55和4.49 eV。这项工作不仅拓宽了亚磷酸酯基材料的结构和组成多样性,而且为开发具有可调性能的功能性亚磷酸酯提供了一种新的设计策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Solid State Chemistry
化学-无机化学与核化学
CiteScore
6.00
自引率
9.10%
发文量
848
审稿时长
25 days
期刊介绍:
Covering major developments in the field of solid state chemistry and related areas such as ceramics and amorphous materials, the Journal of Solid State Chemistry features studies of chemical, structural, thermodynamic, electronic, magnetic, and optical properties and processes in solids.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


