Aluminum salt electrolyte additives with anion insertion/extraction for high-voltage zinc and nickel metal batteries at ultra-low temperatures
IF 20.2
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Compared with divalent metal cations, trivalent Al3+ has a higher hydrolysis constant and exhibits stronger proton dissociation ability, which provides new opportunities for low-temperature energy storage devices. Unfortunately, the high charge density and strong Coulomb interaction of Al3+ limit this application. To address the challenge, this work innovatively used aluminum salt as an electrolyte additive and achieved efficient operation of zinc and nickel metal batteries at ultra-low temperatures by synergistically utilizing the advantages of anions and cations. XPS and in-situ Raman spectroscopy reveal that anions (Cl− and ClO4−) can achieve effective insertion/extraction in the cathode, significantly improving the redox kinetics of the metal battery. Meanwhile, Al3+, which offers a low binding energy of -9.12 eV with water molecules, achieves excellent deposition/stripping reversibility on the metallic Zn and Ni surface. This synergistic effect of anions and cations not only improves the open circuit voltage of the battery, but also significantly reduces the ion diffusion resistance at low temperatures. Consequently, Zn//Zn and Ni//Ni symmetric cells can operate for more than 400 h at -30°C, and the polarization voltage remains highly stable. Impressively, zinc and nickel metal batteries with 2 m AlCl3 as the electrolyte additive are able to maintain voltages of 1.28 V and 0.55 V, respectively, after 550 h of standing at -30°C. The zinc metal battery still has a discharge specific capacity of 118.5 mAh g−1 and a Coulombic efficiency of 96.9 % after 2400 cycles at -50°C. Even under temperature fluctuations from -15 to 5°C, the battery can still maintain a discharge specific capacity of 168.8 mAh g−1. This electrolyte design strategy based on aluminum salt additives is expected to promote the application of energy storage devices in extreme environments.
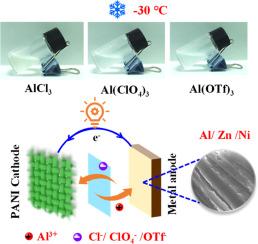
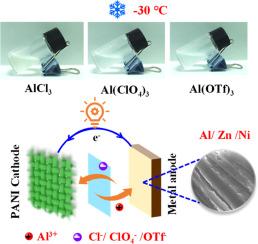
超低温高压锌镍电池用阴离子插入/萃取铝盐电解质添加剂
与二价金属阳离子相比,三价Al3+具有更高的水解常数和更强的质子解离能力,这为低温储能器件提供了新的机遇。遗憾的是,Al3+的高电荷密度和强库仑相互作用限制了它的应用。为了解决这一挑战,本研究创新性地使用铝盐作为电解质添加剂,通过协同利用阴离子和阳离子的优势,实现了锌镍金属电池在超低温下的高效运行。XPS和原位拉曼光谱结果表明,阴离子(Cl−和ClO4−)可以在阴极内实现有效的插入/提取,显著提高金属电池的氧化还原动力学。Al3+与水分子具有-9.12 eV的低结合能,在金属Zn和Ni表面具有优异的沉积/剥离可逆性。这种阴离子和阳离子的协同作用不仅提高了电池的开路电压,而且显著降低了低温下的离子扩散阻力。因此,Zn//Zn和Ni//Ni对称电池可以在-30°C下工作400小时以上,并且极化电压保持高度稳定。令人印象深刻的是,添加2 m AlCl3作为电解液添加剂的锌和镍金属电池在-30°C下放置550 h后,分别能够保持1.28 V和0.55 V的电压。在-50℃下循环2400次后,锌金属电池的放电比容量仍为118.5 mAh g−1,库仑效率为96.9%。即使在-15 ~ 5℃的温度波动下,电池仍能保持168.8 mAh g−1的放电比容量。这种基于铝盐添加剂的电解质设计策略有望推动储能装置在极端环境下的应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Energy Storage Materials
Materials Science-General Materials Science
CiteScore
33.00
自引率
5.90%
发文量
652
审稿时长
27 days
期刊介绍:
Energy Storage Materials is a global interdisciplinary journal dedicated to sharing scientific and technological advancements in materials and devices for advanced energy storage and related energy conversion, such as in metal-O2 batteries. The journal features comprehensive research articles, including full papers and short communications, as well as authoritative feature articles and reviews by leading experts in the field.
Energy Storage Materials covers a wide range of topics, including the synthesis, fabrication, structure, properties, performance, and technological applications of energy storage materials. Additionally, the journal explores strategies, policies, and developments in the field of energy storage materials and devices for sustainable energy.
Published papers are selected based on their scientific and technological significance, their ability to provide valuable new knowledge, and their relevance to the international research community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


