Root specialized metabolites shape plant-beneficial bacterial communities to mitigate hydrocarbon stress
IF 6.9
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
Plant roots secrete various compounds to attract beneficial microbes from soil, enhancing resilience to environmental stresses. The mechanisms by which perennial grasses accumulate specific plant-beneficial bacteria under organic pollution remain unclear. We conducted a pot experiment using ryegrass grown in diesel-contaminated soils (0–15 g/kg) to analyze rhizosphere bacterial community and root exudate, using 16S rRNA gene amplicon sequencing and untargeted metabolomics. A significant increase in the relative abundance of rhizosphere plant-beneficial bacteria was observed along the contamination gradient (r2 = 0.64, p < 0.01), with bacterial taxa possessing dual capabilities in hydrocarbon degradation and nitrogen fixation, such as Nocardioides, becoming more dominant. We identified 22 core metabolites (defined as consistently differential metabolites that showed significant increase across all contamination treatments) including organoheterocyclic compounds, benzenoids, and phenylpropanoids. These core metabolites significantly predicted the dissimilarity of plant-beneficial bacterial communities (Mantel test, r2 = 0.17, p < 0.001). Additionally, we verified the chemotactic response of two plant-beneficial bacterial strains, Rhodopseudomonas sp. and Pseudomonas sp., towards eugenol (a benzenoid), 7-acetoxy-4-methylcoumarin (a phenylpropanoid), and benzil (a benzenoid). Among these, eugenol is a promising compound that selectively induces beneficial microbes, helping ryegrass cope with hydrocarbon stress. These findings advance beyond prior observations of general exudate changes by identifying conserved metabolic responses across contamination levels, and demonstrating their role in microbial recruitment via chemoattraction. Our study provided valuable insights into the potential application of rhizosphere engineering for phytoremediation of hydrocarbon-contaminated soils, as well as the development of microbial-based strategies to enhance crop resilience under environmental stresses.
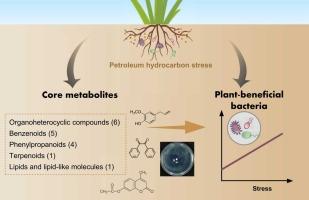
根特化代谢物形成植物有益细菌群落,以减轻碳氢化合物胁迫
植物根系分泌各种化合物,从土壤中吸引有益微生物,增强对环境压力的适应能力。多年生牧草在有机污染条件下积累特定植物有益菌的机制尚不清楚。采用16S rRNA基因扩增子测序和非靶向代谢组学技术,对生长在柴油污染土壤(0-15 g/kg)中的黑麦草根际细菌群落和根系分泌物进行了盆栽试验。根际植物有益菌的相对丰度沿污染梯度显著增加(r2 = 0.64, p <; 0.01),具有碳氢化合物降解和固氮双重能力的细菌类群(如Nocardioides)变得更占优势。我们确定了22种核心代谢物(定义为一致的差异代谢物,在所有污染处理中均显着增加),包括有机杂环化合物、苯类化合物和苯丙类化合物。这些核心代谢物显著预测植物有益菌群落的差异性(Mantel检验,r2 = 0.17, p <; 0.001)。此外,我们还验证了两种有益植物的细菌菌株Rhodopseudomonas sp.和Pseudomonas sp.对丁香酚(一种苯类)、7-乙酰氧基-4-甲基香豆素(一种苯类)和苄基(一种苯类)的趋化反应。其中,丁香酚是一种有前途的化合物,可以选择性地诱导有益微生物,帮助黑麦草应对烃类胁迫。这些发现超越了之前对一般渗出物变化的观察,确定了污染水平上的保守代谢反应,并证明了它们通过化学吸引在微生物招募中的作用。本研究为根际工程在烃类污染土壤植物修复中的潜在应用,以及开发基于微生物的策略来提高作物在环境胁迫下的抗逆性提供了有价值的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Microbiological research
生物-微生物学
CiteScore
10.90
自引率
6.00%
发文量
249
审稿时长
29 days
期刊介绍:
Microbiological Research is devoted to publishing reports on prokaryotic and eukaryotic microorganisms such as yeasts, fungi, bacteria, archaea, and protozoa. Research on interactions between pathogenic microorganisms and their environment or hosts are also covered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


