Hydrothermal upcycling of polyoxymethylene waste on heterogeneous Mn catalyst: The effect of reaction medium
IF 6.3
3区 工程技术
Q1 ENGINEERING, CHEMICAL
Journal of the Taiwan Institute of Chemical Engineers
Pub Date : 2025-08-30
DOI:10.1016/j.jtice.2025.106376
引用次数: 0
Abstract
Background
Polyoxymethylene (POM), an engineering thermoplastic widely used in various applications, is difficult to recycle due to its high stiffness and excellent stability. Hydrothermal conversion has emerged as a promising method for recycling POM, offering moderate operating conditions, reduced harmful emissions, and selective production of valuable chemicals like methanol and formic acid. Recent studies show that catalytic hydrothermal processes can further enhance efficiency and selectivity, though homogeneous catalysts raise concerns about recovery and environmental safety. In contrast, heterogeneous catalysts offer better recyclability and reduce the risk of hazardous waste generation.
Methods
This study proposes a hydrothermal conversion process as a POM recycling method. A Mn catalyst supported on activated carbon (Mn/AC) and different reaction media (N2 and CO2) are applied to the hydrothermal POM conversion as an alternative approach against homogeneous catalytic POM conversion. The effects of reaction temperature (220–260 °C), reaction medium (N2 vs. CO2), and the catalyst presence on the hydrothermal POM conversion are investigated.
Significant Findings
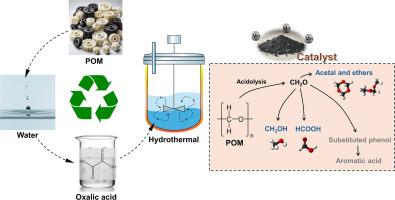
The hydrothermal POM conversion leads to formic acid, methanol, and 1,3,5-trioxane as major reaction products. Formic acid reached its highest yield of 9.79 wt% at 240 °C without the catalyst under N2 atmosphere. Under CO2 atmosphere, methanol production peaked at 1.54 wt% at 260 °C with the Mn/AC catalyst. The highest yield of 1,3,5-trioxane (1.08 wt%) was observed at 220 °C under N2 atmosphere with the Mn/AC catalyst. Therefore, due to the optimal conditions required for each target product, process parameters must be strategically controlled to meet specific production goals. The experimental findings open up the possibility of using heterogeneous catalysts for hydrothermal POM conversion to recover its monomeric compounds.
多相锰催化剂对聚氧甲烷废渣水热回收的影响
聚甲醛(POM)是一种广泛应用的工程热塑性塑料,由于其高刚度和优异的稳定性而难以回收。水热转化已成为回收POM的一种很有前途的方法,它提供了适中的操作条件,减少了有害物质的排放,并选择性地生产有价值的化学品,如甲醇和甲酸。最近的研究表明,催化水热法可以进一步提高效率和选择性,尽管均相催化剂引起了对回收和环境安全的担忧。相比之下,多相催化剂具有更好的可回收性,降低了有害废物产生的风险。方法提出一种水热转化工艺作为POM的回收方法。采用活性炭负载Mn催化剂(Mn/AC)和不同的反应介质(N2和CO2),作为均相催化POM转化的替代方法,应用于水热催化POM转化。考察了反应温度(220 ~ 260℃)、反应介质(N2 vs. CO2)和催化剂用量对水热POM转化的影响。水热法转化POM的主要产物为甲酸、甲醇和1,3,5-三氧环。在无催化剂的情况下,在N2气氛下,甲酸收率在240℃时达到最高,为9.79 wt%。在CO2气氛下,Mn/AC催化剂的甲醇产量在260°C时达到1.54 wt%。在220°C N2气氛下,Mn/AC催化剂下,1,3,5-三氧环的收率最高,为1.08 wt%。因此,由于每个目标产品所需的最佳条件,必须对工艺参数进行战略控制,以满足特定的生产目标。该实验结果开辟了使用多相催化剂进行水热POM转化以回收其单体化合物的可能性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.10
自引率
14.00%
发文量
362
审稿时长
35 days
期刊介绍:
Journal of the Taiwan Institute of Chemical Engineers (formerly known as Journal of the Chinese Institute of Chemical Engineers) publishes original works, from fundamental principles to practical applications, in the broad field of chemical engineering with special focus on three aspects: Chemical and Biomolecular Science and Technology, Energy and Environmental Science and Technology, and Materials Science and Technology. Authors should choose for their manuscript an appropriate aspect section and a few related classifications when submitting to the journal online.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


