BREEZE Optional Extension Phase: Long-term safety and efficacy of treprostinil dry powder inhaler (Tyvaso DPI) in pulmonary arterial hypertension
IF 3.1
3区 医学
Q2 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
Abstract
Introduction
Pulmonary arterial hypertension (PAH) is a rare and progressive disease associated with significant morbidity and mortality. Prostacyclins, including treprostinil, are a mainstay of PAH treatment, particularly in patients with intermediate to high risk of death. Following the approval of treprostinil inhalation solution for PAH, treprostinil dry powder inhaler (DPI) was developed as a small, portable, low-maintenance device to improve patient experience.
Objective
The primary objective of the BREEZE study was to assess the safety and tolerability of treprostinil DPI in PAH.
Methods
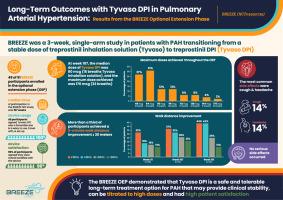
BREEZE was a 3-week, single-arm, open-label study in which patients with PAH transitioned from a stable dose of treprostinil inhalation solution to a comparable dose of treprostinil DPI.
Results
Following the 3-week treatment phase, 49 of 51 patients opted to enroll in the Optional Extension Phase (OEP). Throughout the OEP, 6MWD continued to increase with a median change from baseline of 16 m at week 107 and over a third of patients experiencing an improvement of at least 30 m. Patient satisfaction with the DPI device was overwhelmingly positive while drug-related adverse events were infrequent and characteristic of prostacyclin therapy.
Conclusion
The BREEZE OEP successfully demonstrated the safety of long-term treatment with treprostinil DPI in patients with PAH.
Clinical trial registration
NCT03950739.
BREEZE可选扩展期:替前列地尼干粉吸入器(Tyvaso DPI)治疗肺动脉高压的长期安全性和有效性
肺动脉高压(PAH)是一种罕见的进行性疾病,发病率和死亡率都很高。前列环素,包括曲前列素,是治疗多环芳烃的主要药物,特别是对有中高死亡风险的患者。随着曲前列地尼吸入溶液被批准用于治疗多环芳烃,曲前列地尼干粉吸入器(DPI)作为一种小型、便携、低维护的设备被开发出来,以改善患者的体验。BREEZE研究的主要目的是评估曲前列素DPI治疗PAH的安全性和耐受性。方法sbreeze是一项为期3周的单臂、开放标签研究,在该研究中,PAH患者从稳定剂量的曲前列尼吸入溶液过渡到相当剂量的曲前列尼DPI。结果:在3周治疗期后,51名患者中有49名选择参加可选延长期(OEP)。在整个OEP过程中,6MWD持续增加,在第107周时从基线的中位数变化为16米,超过三分之一的患者至少改善了30米。患者对DPI装置的满意度是压倒性的积极,而药物相关的不良事件是罕见的和特征的前列环素治疗。结论BREEZE OEP成功证明了treprostinil DPI长期治疗PAH患者的安全性。临床试验注册号nct03950739。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Respiratory medicine
医学-呼吸系统
CiteScore
7.50
自引率
0.00%
发文量
199
审稿时长
38 days
期刊介绍:
Respiratory Medicine is an internationally-renowned journal devoted to the rapid publication of clinically-relevant respiratory medicine research. It combines cutting-edge original research with state-of-the-art reviews dealing with all aspects of respiratory diseases and therapeutic interventions. Topics include adult and paediatric medicine, epidemiology, immunology and cell biology, physiology, occupational disorders, and the role of allergens and pollutants.
Respiratory Medicine is increasingly the journal of choice for publication of phased trial work, commenting on effectiveness, dosage and methods of action.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


