N, S Co-doped porous carbons from coconut shell for selective CO2 adsorption
IF 6.2
2区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
Abstract
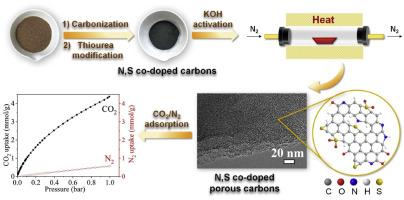
The CO2 capture from flue gas using biomass-derived porous carbons offers a promising and sustainable approach to mitigate greenhouse gas emissions. However, achieving high adsorption performance under ambient conditions requires synergistic optimization of pore architecture and surface chemistry. In the present work, a facile and scalable synthesis method was developed to prepare nitrogen and sulfur co-doped porous carbons using coconut shell as a renewable carbon precursor and thiourea as a dual heteroatom source. Chemical activation with KOH was employed to tune the porosity and surface functionality. The optimal adsorbent exhibited a high BET surface area (1315 m2/g), large narrow micropore volume (0.66 cm3/g), and significant heteroatom content (3.39 at.% N and 0.39 at.% S), resulting in superior CO2 uptake of 4.38 and 6.46 mmol/g at 25 °C and 0 °C, 1 bar, respectively. Additionally, as-prepared adsorbents demonstrated high CO2/N2 selectivity, rapid adsorption kinetics, moderate isosteric heat of adsorption, and excellent cycling stability over five adsorption–desorption cycles. These findings underscore the dual role of narrow micropores and heteroatom-rich functional groups in enhancing gas–solid interactions and provide a green and effective strategy for designing high-efficiency CO2 sorbents from coconut shell waste.
椰壳中N, S共掺杂多孔碳的选择性CO2吸附
利用生物质衍生多孔碳从烟道气中捕集二氧化碳为减少温室气体排放提供了一种有希望的可持续方法。然而,在环境条件下获得高吸附性能需要孔隙结构和表面化学的协同优化。本文以椰子壳为可再生碳前驱体,硫脲为双杂原子源,开发了一种简便、可扩展的制备氮硫共掺杂多孔碳的方法。利用KOH化学活化来调整孔隙度和表面功能。最佳吸附剂具有较高的BET表面积(1315 m2/g)、较大的窄微孔体积(0.66 cm3/g)和显著的杂原子含量(3.39 at。% N和0.39 at。% S),在25°C和0°C, 1 bar时,CO2吸收率分别为4.38和6.46 mmol/g。此外,所制备的吸附剂具有高的CO2/N2选择性,快速的吸附动力学,中等的等等吸附热,以及在5个吸附-脱附循环中优异的循环稳定性。这些发现强调了窄微孔和富杂原子官能团在增强气固相互作用中的双重作用,并为设计高效的椰子壳CO2吸附剂提供了绿色有效的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of The Energy Institute
工程技术-能源与燃料
CiteScore
10.60
自引率
5.30%
发文量
166
审稿时长
16 days
期刊介绍:
The Journal of the Energy Institute provides peer reviewed coverage of original high quality research on energy, engineering and technology.The coverage is broad and the main areas of interest include:
Combustion engineering and associated technologies; process heating; power generation; engines and propulsion; emissions and environmental pollution control; clean coal technologies; carbon abatement technologies
Emissions and environmental pollution control; safety and hazards;
Clean coal technologies; carbon abatement technologies, including carbon capture and storage, CCS;
Petroleum engineering and fuel quality, including storage and transport
Alternative energy sources; biomass utilisation and biomass conversion technologies; energy from waste, incineration and recycling
Energy conversion, energy recovery and energy efficiency; space heating, fuel cells, heat pumps and cooling systems
Energy storage
The journal''s coverage reflects changes in energy technology that result from the transition to more efficient energy production and end use together with reduced carbon emission.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


