Characterizing the symbiotic relationship between Wolbachia (wSpic) and Spodoptera picta (Lepidoptera: Noctuidae): From genome to phenotype
IF 3.7
2区 农林科学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Wolbachia is a genus of symbiotic bacteria prevalent in arthropods, with diverse effects on host reproduction and fecundity; however, it is unclear how Wolbachia modulates the host reproductive system. In this study, a novel Wolbachia strain, wSpic, was identified in the Noctuid moth Spodoptera picta and its effect on the reproduction of this host was investigated. We sequenced and annotated the 1,339,720 bp genome of wSpic. We identified a total of five WO phage regions in the genome and found no evidence of any plasmids associated with wSpic. Evolutionary analysis revealed that wSpic belongs to supergroup B and has undergone horizontal transmission between S. picta and Trichogramma pretiosum, a wasp parasitoid of insect eggs. The removal of Wolbachia by antibiotic treatment resulted in significantly decreased fecundity and abnormal development of S. picta ovaries, but no differences in egg hatching rate. An integrated transcriptome and proteome analysis indicated that major molecular pathways for Wolbachia-induced reproduction fitness benefits include its effects on insect juvenile hormone, vitellogenesis, choriogenesis, and nutritional metabolism. Our findings demonstrate that wSpic plays a critical role in promoting ovary development and sustaining fecundity in S. picta hosts.
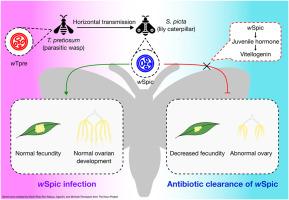
沃尔巴克氏体(wSpic)与象形夜蛾(鳞翅目:夜蛾科)共生关系的表征:从基因组到表型
沃尔巴克氏体是一种在节肢动物中普遍存在的共生细菌属,对宿主的繁殖和繁殖力有不同的影响;然而,目前尚不清楚沃尔巴克氏体如何调节宿主的生殖系统。本研究在夜蛾(Spodoptera picta)中鉴定出一种新的沃尔巴克氏体菌株wSpic,并对其对夜蛾繁殖的影响进行了研究。我们对wSpic的1,339,720 bp基因组进行了测序和注释。我们在基因组中共鉴定了5个WO噬菌体区域,没有发现任何与wSpic相关的质粒的证据。进化分析表明,wSpic属于超B群,并在picta和蜂卵寄生蜂Trichogramma pretiosum之间进行了水平传播。抗生素处理去除沃尔巴克氏体后,雌蜂卵巢繁殖力明显下降,发育异常,但卵孵化率无显著差异。综合转录组和蛋白质组分析表明,沃尔巴克氏体诱导的生殖适应性益处的主要分子途径包括其对昆虫幼体激素、卵黄形成、绒毛膜形成和营养代谢的影响。我们的研究结果表明,wSpic在促进picta寄主卵巢发育和维持繁殖力方面起着关键作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.40
自引率
5.30%
发文量
105
审稿时长
40 days
期刊介绍:
This international journal publishes original contributions and mini-reviews in the fields of insect biochemistry and insect molecular biology. Main areas of interest are neurochemistry, hormone and pheromone biochemistry, enzymes and metabolism, hormone action and gene regulation, gene characterization and structure, pharmacology, immunology and cell and tissue culture. Papers on the biochemistry and molecular biology of other groups of arthropods are published if of general interest to the readership. Technique papers will be considered for publication if they significantly advance the field of insect biochemistry and molecular biology in the opinion of the Editors and Editorial Board.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


